1918
Detecting Synaptic Dysfunction caused by Pathological α-Synuclein Accumulation in Mouse Brain using MRS
Yuhei Takado1, Maiko Ono2, Keiichiro Minatohara2, Masafumi Shimojo2, Nobuhiro Nitta2, Sayaka Shibata2, Naruhiko Sahara2, Ichio Aoki2, Masato Hasegawa3, and Makoto Higuchi2
1Department of Functional Brain Imaging Research, National Institutes for Quantum and Radiological Science and Technology, Chiba, Japan, 2National Institutes for Quantum and Radiological Science and Technology, Chiba, Japan, 3Dementia Research Project, Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan
1Department of Functional Brain Imaging Research, National Institutes for Quantum and Radiological Science and Technology, Chiba, Japan, 2National Institutes for Quantum and Radiological Science and Technology, Chiba, Japan, 3Dementia Research Project, Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan
Synopsis
To develop therapeutic strategies, in vivo detection of the early pathological changes in Parkinson’s disease is critically important. In this work, we aimed to detect early pathological changes caused by the propagation of α-synuclein in mouse brain using MRS. Recombinant α-synuclein and fibrils were prepared and injected into C57BL6 mice. 8 weeks after the injection, glutamate levels were decreased significantly compared to saline-injected control mice, which was in accordance with decreased synapsin staining in the cortex. We demonstrated that MRS can detect synaptic dysfunction caused by α-synuclein propagation in vivo.
Introduction
Parkinson's disease (PD) and related disorders are characterized by filamentous structures consisting of abnormal α-synuclein in the brains. The propagation of these pathologies is closely correlated with disease progression, and synaptic dysfunction caused by these pathologies is considered to be an early event during the disease progression1. To develop therapeutic strategies, in vivo detection of the early pathological alterations is critically important. Proton magnetic resonance spectroscopy (MRS) is a non-invasive method that can detect neurotransmitters such as glutamate (Glu). At the resting state under anesthesia, Glu levels measured by MRS (MRS-measured Glu) are known to reflect synaptic densities in the pathological brains2. In this work, we aimed to detect early pathological changes caused by accumulation of α-synuclein in vivo using MRS. We hypothesized that MRS-measured Glu can detect an early pathological change, namely synaptic dysfunction, in the α-synuclein accumulation mouse model.Methods
Mouse Recombinant mouse α-synuclein and fibrils were prepared as described previously3. 12 C57BL/6 wild type male mice were used for this experiment (6 for α-synuclein injection, and 6 for saline injection as control). Under anesthesia with ketamine hydrochloride (71.5 mg/kg, i.p.) and xylazine (14.5 mg/kg, i.p.), the animals were injected with 4 mL of 4 mg/mL mouse α-synuclein fibrils into the bilateral cortex, consecutively. After the MRS measurements described below, the brain was perfused with saline and fixed in 4% PFA in PBS. After cryo-protection in PBS containing 20% sucrose, brains were embedded and frozen in OCT compound, and 20-mm thick sections were prepared by cryostat. Histological analysis: Immunostaining was performed on scanned mice with NeuN and synapsin antibodies for staining of neurons and presynapses, respectively.MRI and 1H-MRS Both α-synuclein fibril- and saline-injected mice were scanned twice by MRI, both before and 8 weeks after the injections. For MRS measurements, the mice were anesthetized with 1–2% isoflurane and scanned by a 7 T spectrometer (Biospec, AVANCE-III, Bruker Biospin) with a dual-channel phased-array cooled surface coil for transmission and reception (cryoprobe©, Bruker Biospin) using a PRESS sequence (TR/TE = 4000/20 ms). Two volume of interests (VOIs) were localized in the right and left cortex. 256 acquisitions were collected for each cortex. Using total creatine (tCr, the sum of phosphocreatine (PCr) and Cr) signal as a reference, metabolite concentration ratios were calculated using LCModel. In this work, we focused on Glu and total N-acetylaspartate (tNAA, the sum of N-acetylaspartate and N-acetylaspartylglutamate) to test our hypothesis.
Statistical analyses Results were presented as mean ± standard error of the mean. For multiple group comparisons, one-way ANOVA was performed, followed by Fisher’s least significant difference post-hoc test.
Results
Eight weeks after the injection of α-synuclein fibrils, the levels of Glu/tCr were reduced significantly compared to saline-injected mice. There was also significant decrease of tNAA in the α-synuclein fibril-injected mice compared to saline-injected mice. Histological analysis indicated that α-synuclein deposition was propagated from the injected site to the neighboring regions. Synapsin staining demonstrated that presynapses were damaged by α-synuclein accumulation. NeuN staining did not show evidence of neuronal loss, which is in accordance with the currently proposed pathophysiological claim that synaptic dysfunction precedes neurodegeneration1.Discussion
In this work, we investigated whether MRS-measured Glu detects synaptic dysfunction caused by pathological α-synuclein accumulation in mouse brain. MRS-measured Glu levels were significantly decreased compared to control mice, suggesting that synaptic dysfunction leads to decreased Glu levels in presynaptic sites. Histological analysis was in agreement with this interpretation, since synapsin protein exists mainly at presynaptic terminals4. No evidence of NeuN alterations suggests that neuronal loss has not appeared at this stage. Decreased tNAA levels without evident neuronal loss confirmed by histological analysis may indicate that axonal impairments contributed to the decrease of tNAA, since tNAA is known to exist not only in soma but also in axons5. Mitochondrial dysfunction may also be a potential mechanism of decreased tNAA.Conclusion
We demonstrated that MRS can detect synaptic dysfunction caused by pathological α-synuclein accumulation in vivo. This method using MRS would be valuable for evaluating therapeutic efficacies of various potential drugs to develop therapeutic strategies for α-synucleinopathies such as Parkinson’s disease and other related disorders.Acknowledgements
We acknowledge Ms. Shoko Uchida, Mr. Takahiro Shimizu, Mr. Takeharu Minamihisamatsu and other colleagues for valuable help for our experiments.References
1. Frontiers in neuroscience 2018;12:80. 2. NeuroImage 2014;101:185-192. 3. Acta neuropathologica communications 2014;2:88. 4. The Journal of neuroscience : the official journal of the Society for Neuroscience 2004;24:3711-3720. 5. Progress in neurobiology 2007;81:89-131.Figures
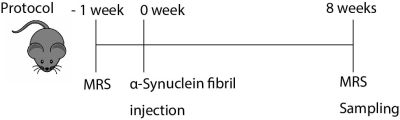
Fig.1 Protocol of
the experiment
Schematic
diagram of the experimental protocol of this study is shown. MRS measurements
were performed before and 8 weeks after α-Synuclein fibrils injections.
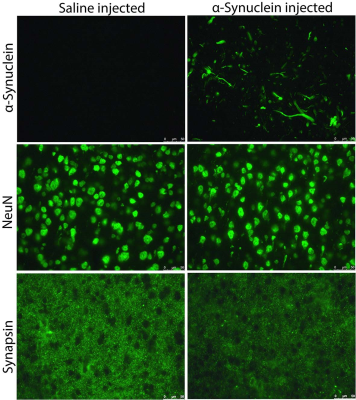
Fig.2 Immunostaining
Phosphorylated
α-Synuclein staining was positive in α-Synuclein fibril-injected mouse, and no
staining was found in saline-injected mouse. There was no noticeable difference
in NeuN staining between the two mice. Synapsin staining in the α-Synuclein
fibril-injected mouse was decreased compared to the saline-injected mouse.
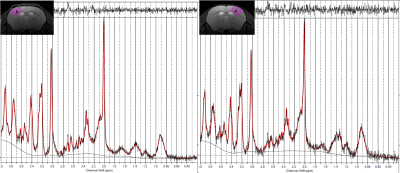
Fig.3 Representative
voxel of interests (VOIs) in the right and left cortex and their spectra
Representative
VOIs and spectra of the left and right cortex are shown.
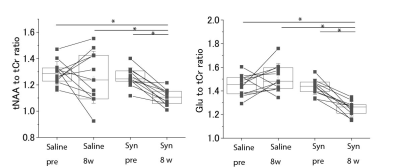
Fig.4 Results of MRS
measurements
The
results of the tNAA to tCr and Glu to tCr ratios of MRS measurements are shown.
While there were no significant differences in tNAA and Glu between before
injection (pre) and 8 weeks after the injection (8w) of saline, there were significant
differences in the case of α-Synuclein fibril injection. * p < 0.05 as
analyzed by one-way ANOVA.