1917
Quantitative white matter fibers in a mouse model of a neurodevelopmental disease: Insights from high spatial resolution 3D-DTI1BrainTech Lab Inserm U1205, Grenoble, France, 2GIN Inserm U836, Grenoble, France, 3iBV Inserm U1091, Nice, France
Synopsis
Microscopic 3D-DTI was applied to detect brain connection defects in COUP-TFI-mutant mice. Several tractography abnormalities were identified supporting a major role of COUP-TFI gene acting in the formation and guidance of forebrain commissures. DTI results are in agreement with those using fluorescent dyes, but identifies deficiencies of other cortical tracts not previously described. As COUP-TFI (NR2F1 in humans) mutations were also linked to a complex neurodevelopmental disease in humans, this work underlines the interest of 3D-DTI to study the whole brain in patients as those affected with Bosch-Boonstra-Schaaf optic-atrophy syndrome due to NR2F1 gene mutations/deletions, an emerging rare neurodevelopmental disease.
Introduction
In mice, COUP-TFI gene (named NR2F1 in humans), was shown to play a key role in cortical development, particularly in cell specification and migration, axogenesis and connectivity (1-2). In previous studies on COUP-TFI-mutant mice, in which the gene is inactivated, histological analysis and axonal tracing with fluorescent dyes have shown various defects in the pathway of the major brain commissural projections (3-4). Since individual sections are not sufficient to obtain a reliable 3D view of axonal projections, we propose here to use Diffusion Tensor Imaging (DTI), an ideal tool to characterize neuroanatomical tract defects of whole brains. Our aim is to identify defective axonal tracts in mice lacking the COUP-TFI gene, an important task to contribute to our understanding of the COUP-TFI/NR2F1 role in normal and pathological brain development. By analyzing the data obtained by 3D-DTI fiber-tracking, we aim not only to support histological axonal tracing obtained using fluorescent dyes (3-4) but also to identify new defects that can be further analyzed by cellular and molecular means.Methods
The COUPE-TFI constitutive and conditional mouse lines were obtained using the Cre-lox technology as previously described (3,5). All experiments were conducted ex vivo (COUP-TFI-mutant n=6 and Wild-Type (WT): n=4). Brains were fixed by transcardiac perfusion of paraformaldehyde 4% solution containing 6.25mM of Gd-DOTA, a paramagnetic contrast agent. After removing surrounding skin and muscles, the skulls with intact brains were immersed in the same solution during 4 days and then after conserved in Fomblin-oil at 4°C, 11 days before MRI. Gd-DOTA was used to reduce T1 relaxation for MRI scan acceleration. MRI were performed on a Bruker 9.4T (Biospec 94/20) equipped with a gradient-system (900mT/m) and a RF Cryo-coil, using microscopic 3D-DTI of (80µm×80µm×80µm) isotropic spatial resolution in a FOV of 15mm×12mm×10mm. The Stejskal-Tanner spin-echo sequence with repetition-time/eho-time/echo,TR/TE=90ms/8ms and gradient durations (δ=4ms, Δ=8ms) was applied in six spatial directions ([1 1 0], [1 -1 1], [0 1 1], [0 1 -1], [1 0 1], [-1 0 1]) with a b-value of 1700s.mm-2 and number of accumulations NA=4. 3D-DTI data were processed using Tensor-Toolkit software (https://gforge.inria.fr/projects/ttk) for reconstruction with initial and cutoff fractional anisotropy values of FA1=0.4 and FA2=0.28 respectively. MedINRIA software was used for visualization and tract quantifications (for details see (6)). Tract defects of COUP-TFI-mutant mice were analyzed by comparing their mean volume of fibers per tract to those of WT. The fasciculus-retroflexus (FR) tract was found unchanged and used as an internal reference. Quantitative volume (V) changes were obtained using the equation: [<V(tract)Mutant>/<V(FR)Mutant>]/[< V(tract)WT>/<V(FR)WT>]×100 , where the symbol < > represents the mean value.Results
Representative reconstructions of some key neuronal tracts illustrating specific alterations in COUP-TFI- mutant mice are shown in (Fig.1). Abnormal branching and fasciculation are observed in numerous tracts along with overall reduced volumes. This is the case of the corpus-callosum (CC) and the fornix (F), for which abnormalities are also clearly visible in T2W images (Fig.2). Moreover, COUP-TFI loss of function also results in an aberrant volume increase of the anterior-commissure (AC) and the pyramidal (PY) tracts (Fig.3), indicating that COUP-TFI is required for the correct positioning and guiding of these tract bundles. The deficiencies observed in the (CC) and in the ventral-hippocampal-commissure (VHC) are in line with those previously described (2) and confirm that all of major forebrain commissures have guidance and volume abnormalities along the anterior-posterior axis. However, DTI fiber-tracking have also disclosed novel deficiencies of other tracts, such as the stria-medullaris (SM), and the stria-terminalis (ST), and clearly and systematically show absence of commissural tracts despite the presence of axonal fibers. Indeed, as we can see, at the junction of the right and left hemispheres, the (VHC) tract is present but incomplete in mutant brains.Discussion/Conclusion
In this study, we show that high spatial resolution 3D-DTI tractography is really useful for the visualization of white matter structures in the whole mouse brain, detecting major defects in brain connections, in accordance with previous studies using histological axonal tracing with markers. In the absence of COUP-TFI, our results highlight tractography abnormalities and several differences in neuroanatomical volumes and confirm the COUP-TFI role in the formation and guidance of forebrain commissures. Deficiencies of two novel tracts (SM), and (ST) were detected by 3D-DTI and should contribute in our understanding of the COUP-TFI/NR2F1 role in brain development. Differently to neuronal tracer using fluorescent dyes, 3D-DTI could be extended in clinics, in patients with NR2F1 haploinsufficiency as those affected with Bosch-Boonstra-Schaaf optic atrophy syndrome (7-8), since this technique has the unique advantage to study tractography of the whole brain noninvasively.Acknowledgements
We thank Sandra Pierre for her help in brain preparations and Mellila Bouaouali for her participation in DTI experiments and analysis.References
1- Bertacchi, M., Parisot, J., Studer, M. The pleiotropic transcriptional regulator COUP-TFI plays multiple roles in neural development and disease. Brain Res. (2018) https://doi.org/10.1016/j.brainres.2018.04.024
2- Adam, F. et al. COUP-TFI (chicken ovalbumin upstream promoter-transcription factor I) regulates cell migration and axogenesis in differentiating P19 embryonal carcinoma cells. Mol. Endocrinol. 14, 1918–1933 (2006).
3- Armentano M, Filosa A, Andolfi G and Studer M. COUP-TFI is required for the formation of commissural projections in the forebrain by regulating axonal growth. Development 133, 4151-4162 (2006)
4- Bertacchi, M., Gruart, A., Kaimakis, P., Allet, C., Serra, L., Giacobini, P., Delgado‐García, J.M., Bovolenta, P., Studer, M.Mouse Nr2f1 haploinsufficiency unveils new pathological mechanisms of a human optic atrophy syndrome. EMBO Mol. Med. (2019)https://doi.org/10.15252/emmm.201910291
5- Armentano, M., Chou, S.-J., Srubek Tomassy, G., Leingärtner, A., O’Leary, D.D.M., Studer, M., 2007. COUP-TFI regulates the balance of cortical patterning between frontal/motor and sensory areas. Nat. Neurosci. 10, 1277–1286. https://doi.org/10.1038/nn1958
6- Gimenez U, Boulan B, Mauconduit F, Taurel F, Denarier E, Brocard J, Gory-Fauré S, Andrieux A, Deloulme JC and Lahrech H. 3D imaging of the brain morphology and connectivity defects in a model of psychiatric disorders: MAP6-KO mice". Scientific Reports 2017 4;7(1):10308.
7- Bosch, D.G.M and al. NR2F1 Mutations Cause Optic Atrophy with Intellectual Disability. Am. J. Hum. Genet. 94, 303–309 (2014)
8- Chun-An Chen et al. The expanding clinical phenotype of Bosch-Boonstra-Schaaf optic atrophy syndrome: 20 new cases and possible genotype–phenotype correlations. Genetic in Medicine 18:1143-1150 (2016)
Figures
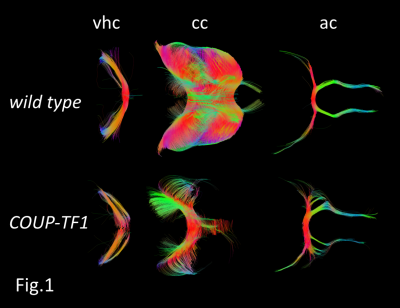

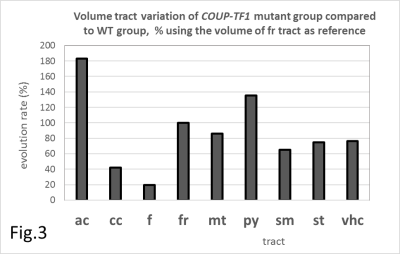
Tract volume changes of COUP-TF1-mutant mice versus WT.
The (FR) tract is used as an internal reference. Quantifications are given according to [<V(tract)Mutant>/<V(FR)Mutant>]/[< V(tract)WT>/<V(FR)WT>]×100