1916
Investigating the Biological Basis of the BOLD fMRI Signal in Mice1ETH Zurich, Zurich, Switzerland, 2University of Zurich, Zurich, Switzerland
Synopsis
How is the BOLD fMRI signal generated still remains unclear. By using a combination of simultaneous calcium imaging and fMRI, we studied the involvement of both astrocytes and neurons in blood flow regulation during electrical hindpaw stimulation in mice. The observed response duration varies based on the anesthesia used, however a strong correlation between astrocytic calcium and BOLD signal was present. Our method allows the study of neurovascular coupling mechanisms in the intact brain and points to the importance of astrocytes in this process, and can be further used to study BOLD signal alterations e.g. in models of neurodegeneration.
Introduction and Aim
Functional magnetic resonance imaging (fMRI) is a technique based on local changes in blood flow and metabolism, and is widely used in research and clinical practice. At the same time, the biological mechanism underlying the generation of blood oxygen-level dependant (BOLD) fMRI signal remains unclear. The neurovascular unit consists of neurons, astrocytes and cells of the surrounding blood vessels that orchestrate together the control of local blood supply. While it has been shown that neurons can independently regulate blood flow (1), this relationship is not always straightforward (1,2). On the other hand, astrocytes have been proposed to couple neuronal activity to changes in cerebrovascular blood flow by the release of vasoactive substances. However, how those two elements of the neurovascular unit are coupled together remains an open question.Methods
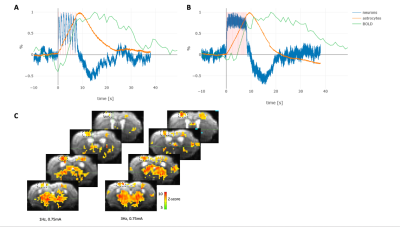
In order to investigate this further, we measured simultaneously calcium activity of astrocytes and neurons in the mouse somatosensory cortex with the use of genetically encoded calcium indicators (GECIs), while at the same time performing fMRI. For the purpose, the right hindpaw area of the somatosensory mouse cortex was transfected by local injection of adeno-associated viral vector encoding GECIs following the procedure previously described (3,4). The use of RCaMP as neuronal and GCaMP as astrocytic calcium reporter allowed for simultaneous detection of activity related signals by using laser excitation at 488 and 561 nm delivered via an optic fiber (4). Emanating fluorescent signals were separated with bandpass filters and detected using photomultiplier tubes. Simultaneously, fMRI signals were recorded using a Bruker 7T Pharmascan equipped with a cryogenic receiver coil (4). For imaging experiments, animals were anesthetized with either isoflurane (1.5%) or a mixture of ketamine-xylazine following a previously published protocol (5), while we performed electrical hindpaw stimulation. The collected optical data was filtered, downsampled and convolved with the hemodynamic response function (HRF) to be used as a regressor in the fMRI analysis.Results
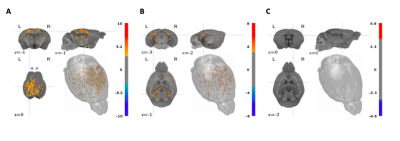
Our results revealed that under isoflurane anesthesia the onset and dynamic of astroglial calcium signal was better correlated with the BOLD signal compared to the neuronal calcium signal, under the relatively mild stimulation conditions used (Fig. 1). We also compared the correlation between BOLD signal and astroglial and neuronal calcium activity in mice anesthetized with ketamine-xylazine mix (5), as a way to circumvent the vasodilatory and non-specific BOLD effect of isoflurane. Similarly, for ketamine-xylazine anesthetized mice, the astrocytic response corresponded better to the BOLD signal as compared to the neuronal one. Both the shape of the calcium traces and the BOLD fMRI response varied based on the anaesthetic used. Moreover, regressor-wise statistical maps yielded a better correlation between the BOLD signal derived from the stimulation protocol convolved with the HRF (classical BOLD signal) and the one obtained using the astroglial calcium trace as a model function, as between the classical BOLD signal and the one derived when using the neuronal signal as model function (Fig. 2).Conclusion
This multimodal approach combining fluorescence fiberscopy yielding information on neuronal and astrocytic calcium traces and fMRI recordings allows the simultaneous monitoring of key components of the neurovascular unit in the intact brain, allowing the study of neurovascular coupling mechanisms. Our data revealed an important role of astrocytes in blood flow control. Targeted manipulation using pharmacological and genetic interventions might provide important insights in the specific roles of the individual constituents (neurons, astrocytes, vasculature). This method could be used for studying pathological alterations in blood regulation, for example in models of neurodegeneration to provide a better understanding of the altered fMRI response.Acknowledgements
No acknowledgement found.References
1. Schulz K, Sydekum E, Krueppel R, et al. Simultaneous BOLD fMRI and fiber-optic calcium recording in rat neocortex. Nat Methods. 2012;9(6):597-602. doi:10.1038/nmeth.2013
2. Arne E. How and when the fMRI BOLD signal relates to underlying neural activity: The danger in dissociation. Brain Res Rev. 2013;62(2):233-244. doi:10.1016/j.brainresrev.2009.12.004.How
3. Stobart JL, Ferrari KD, Barrett MJP, et al. Cortical Circuit Activity Evokes Rapid Astrocyte Calcium Signals on a Similar Timescale to Neurons. Neuron. 2018;98(4):726-735.e4. doi:10.1016/j.neuron.2018.03.050
4. Schlegel F, Sych Y, Schroeter A, et al. Fiber-optic implant for simultaneous fluorescence-based calcium recordings and BOLD fMRI in mice. Nat Protoc. 2018;13:840. https://doi.org/10.1038/nprot.2018.003.
5. Shim H-J, Jung WB, Schlegel F, et al. Mouse fMRI under ketamine and xylazine anesthesia: Robust contralateral somatosensory cortex activation in response to forepaw stimulation. Neuroimage. 2018;177:30-44. doi:10.1016/j.neuroimage.2018.04.062
Figures