1910
A machine learning model using T2-weighted FLAIR radiomics features to predict patient outcome in ICH1Radiology, University of Cincinnati, Cincinnati, OH, United States, 2The Perinatal Institute and Section of Neonatology, Perinatal and Pulmonary Biology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, United States, 3Electrical Engineering and Computer Science, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, United States, 4Pediatrics, University of Cincinnati, Cincinnati, OH, United States, 5Neurology and Rehabilitation Medicine, University of Cincinnati, Cincinnati, OH, United States
Synopsis
Intracerebral hemorrhage (ICH) accounts for 10% - 30% of all strokes and is associated with high short-term mortality (≤50% at 3 month). There is a critical unmet need for an effective prognostic tool using imaging markers to identify patients at risk for poor outcome and thereby better facilitating treatments at individual level as well as tailoring personalized interventions and optimizing rehabilitation strategies. In this work, we developed a machine learning method using radiomics features derived from T2-weighted FLAIR images to predict recovery outcome in patients with ICH at 3 months with a accuracy of 80.8% (95% confidence interval: 78.9%, 82.8%).
Introduction
Intracerebral hemorrhage (ICH) accounts for 10% - 30% of all strokes and is associated with high short-term mortality (≤50% at 3 month). More than one third of survivors suffer severe disability or end up with death 3 months later.1 There is a critical unmet need for an effective prognostic tool using imaging markers to identify patients at risk for poor outcome and thereby better facilitating treatments at individual level to limit ICH growth as well as tailoring personalized interventions and optimizing rehabilitation strategies. Previous studies have reported the use of clinical and imaging features to predict structural outcomes (e.g. hematoma location and enlargement), and functional outcomes estimated by the modified Rankin Scale (mRS).2-6 More recently, radiomics features based on CT images have been reported as outcome predictors in patients with ICH.7 However, outcome prediction studies based on MRI radiomics features are limited. In this study, we aim to develop a machine learning method using radiomics features derived from T2-weighted fluid-attenuated inversion recovery (FLAIR) images to predict recovery outcome in patients with ICH at 3 months.Method
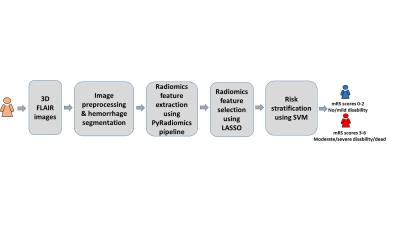
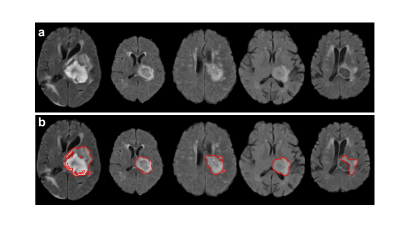
We utilized a convenience sample of 53 left thalamocapsular ICH patients (hemorrhagic volume < 20cc; mean age = 52.4 yrs) from the NIH funded, multicenter Ethnic/Racial Variation Intracerebral Hemorrhage (ERICH) study. The T2-weighted FLAIR data were acquired using standard of care clinical protocols. We considered the patients with 3 month mRS scores 0-2 as favorable outcomes; while those with mRS scores 3-6 as unfavorable outcomes. The overview of the proposed machine learning approach is shown in Figure 1. We conducted following schema for FLAIR data preprocessing: 1) skull stripping (comprises the process of removing skull, extra-meningeal and non-brain tissues from the MRI data); 8 2) bias field correction (reducing the signal intensity inhomogeneity mainly caused by radiofrequency coils); 9 and 3) intensity normalization (reducing the variations of signal intensity and contrast across subjects). 10 We implemented 3D U Net convolutional neural network model to segment hemorrhages, which was originally designed for a volumetric segmentation. 11 The representative hemorrhage segmentation results from five unique patients are shown in Figure 2. We then extracted 105 radiomics features from the segmented hemorrhages using pyRadiomics pipeline,12 including 13 geometric features (e.g., volume, surface area, compactness, maximum diameter, sphericity), 18 histogram features (e.g., variance, skewness, kurtosis, uniformity, entropy), 14 texture features from the Gray-Level Dependence Matrix, 23 texture features from the Gray-Level Co-Occurrence Matrix, 16 texture features from the Gray-Level Run-Length Matrix, 16 texture features from the Gray-Level Size Zone Matrix, and 5 texture features from the Neighborhood Gray-Tone Difference Matrix. To prevent model overfitting, we reduced feature dimensionality using least absolute shrinkage and selection operator (LASSO) algorithm.13 The LASSO algorithm minimizes the residual sum of squares and poses a constraint to the sum of the absolute values of the coefficients being less than a constant. The LASSO algorithm constructs a linear model, which penalizes the coefficients with an L1 penalty, such that some coefficients can be shrunk to zero. Features with non-zero regression coefficients were selected by the LASSO. Based on the 54 LASSO-selected radiomics features, we developed a support vector machine (SVM) model 14 with a polynomial kernel to conduct two-class classification. Using a 5-fold cross-validation, the classification diagnostic performance of our SVM models was established, including accuracy, sensitivity, specificity, and area under the receiver operating characteristic curve (AUC).Result
Our model was able to correctly identify patients likely to have unfavorable outcomes with an accuracy of 80.8% (95% confidence interval: 78.9%, 82.8%), AUC of 0.81 (0.79, 0.83), sensitivity of 88.2% (86.1%, 90.4%) and specificity of 72.7% (69.0%, 76.4%).Discussion and conclusion
In this study, we present an SVM machine learning model using radiomics features derived from T2-weighted FLAIR images to identify ICH patients likely to have unfavorable outcomes. We built the SVM in a supervised fashion from an input dataset (patients with ICH who have 3-month outcomes) with a set of prognostic radiomics features. The constructed model is able to evaluate whether a new patient belongs to no/mild disability (mRS 0-2) or moderate/severe disability group (mRS 3-6). The results of our SVM model are promising, however, further continued refinement with larger internal and external datasets are needed to validate our current model. Additionally, studies are needed to compare our current traditional machine learning approach to deep learning approaches.Acknowledgements
References
1. Feigin, V.L., Lawes, C.M.M., Bennett, D.A. & Anderson, C.S. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. The Lancet Neurology 2, 43-53 (2003).
2. Rodriguez-Luna, D., et al. Multiphase CT angiography improves prediction of intracerebral hemorrhage expansion. Radiology 285, 932-940 (2017).
3. Sporns, P.B., Kemmling, A., Minnerup, J., Hanning, U. & Heindel, W. Imaging-based outcome prediction in patients with intracerebral hemorrhage. Acta Neurochirurgica 160, 1663-1670 (2018).
4. Kidwell, C.S., et al. Ischemic lesions, blood pressure dysregulation, and poor outcomes in intracerebral hemorrhage. Neurology 88, 782-788 (2017).
5. Sporns, P.B., et al. Computed tomographic blend sign is associated with computed tomographic angiography spot sign and predicts secondary neurological deterioration after intracerebral hemorrhage. Stroke 48, 131-135 (2017).
6. Al-Shahi Salman, R., et al. Absolute risk and predictors of the growth of acute spontaneous intracerebral haemorrhage: a systematic review and meta-analysis of individual patient data. The Lancet Neurology 17, 885-894 (2018).
7. Qiu, W., et al. Radiomics-based intracranial thrombus features on CT and CTA predict recanalization with intravenous alteplase in patients with acute ischemic stroke. American Journal of Neuroradiology 40, 39-44 (2019).
8. Jenkinson, M., Pechaud, M. & Smith, S. BET2: MR-based estimation of brain, skull and scalp surfaces. in Eleventh annual meeting of the organization for human brain mapping, Vol. 17 167 (Toronto., 2005).
9. Çiçek, Ö., Abdulkadir, A., Lienkamp, S.S., Brox, T. & Ronneberger, O. 3D U-Net: learning dense volumetric segmentation from sparse annotation. in International conference on medical image computing and computer-assisted intervention 424-432 (Springer, 2016).
10. van Griethuysen, J.J.M., et al. Computational radiomics system to decode the radiographic phenotype. Cancer Research 77, e104-e107 (2017).
11. Kukreja, S.L., Löfberg, J. & Brenner, M.J. A least absolute shrinkage and selection operator (lasso) for nonlinear system identification . IFAC Proceedings Volumes 39, 814-819 (2006).
12. Cortes, C. & Vapnik, V. Support-vector networks. Machine learning 20, 273-297 (1995).
Figures