1906
Detection of cerebral infarction and estimation of vascular territory via deep convolutional autoencode1Department of Radiological Sciences, Graduate School of Human Health Sciences, Tokyo Metropolitan University, Tokyo, Japan, 2Department of Radiology, Graduate School of Medicine, Juntendo University, Tokyo, Japan, 3Department of Radiology, Toho University Omori Medical Center, Tokyo, Japan
Synopsis
In the treatment of acute infarction, the detection of abnormal high signals in diffusion weighted images contributes to early diagnosis and treatment of infarction. In this study, we developed a deep learning neural network model via autoencoder (AE) to diagnosis brain infarction and predict vascular territory automatically from a DWI image. 1582 brain images including normal and abnormal brain which had infarctions were used as a training and test dataset. As a result, our model detected brain infarction and estimated vascular territory with high accuracy. It can be an effective indicator for diagnosing correctly infarction and predicting treatment effect.
INTRODUCTION
Stroke is the second cause of death in the world.1 Stroke imaging has been required for urgent treatment, because brain tissue is rapidly damaged as stroke progresses. That is why it is important that stroke is assessed as soon as possible. Diffusion weighted imaging (DWI) can visualize hyperacute brain infarction, within few minutes after the onset of ischemia, earlier than computed tomography. DWI reflects sensitively diffusion restriction caused by serious restriction of water in affected brain tissue.2 Until now, there is no study which assesses brain infarction and predicts responsible vascular territory automatically from DWI image. In this study, we tried detecting only abnormal signal using a deep neural network model based on unsupervised learning trained with only “normal” brain images, and diagnosing brain infarction from DWI image. Furthermore, we also attempted identifying responsible vascular territory.METHODS
Data Acquisition and Pre-Processing1582 MR images of 1282 normal brains without any neurological and structural abnormalities (age range from 23 to 77 years old, mean 56.4 years old) and 300 abnormal brains with infarct (age range from 44 to 94 years old, mean 72.6 years old) were included. Whole brain DWI was acquired using a 3.0-T (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) with the following parameters: repetition time/echo time = 60/6000ms, NEX = 3, ETL = 38, b = 1000s/mm2, FOV = 230×266mm, matrix = 186×130, slice thickness 2.5mm. These images were converted from digital imaging and communications in medicine (DICOM) in bitmap, and resized to resolution of 256×256. An expert neuroradiologist assessed brain infarction and responsible vascular territory. Furthermore, data was randomly divided into a training dataset accounting for 80 % of whole data (1184 normal brains, 256 abnormal brains; 47 anterior cerebral artery (ACA) territories, 142 middle cerebral artery (MCA) territories, 67 posterior cerebral artery (PCA) territories) and a test dataset accounting for 20 % of whole data (98 normal brains, 44 abnormal brains; 5 ACA territories, 28 MCA territories, 11 PCA territories).
Deep Learning architecture
A combination of two machine learning models was applied to detect and diagnose infarct, and identify responsible vascular territory. These models were constructed using SONY neural network console ver. 1.50 (https://dl.sony.com/) on a Windows PC coming with Intel Corei7 2.20GHz, 32GB memory and graphical processing unit NVIDIA GeForce GTX 1070. The following solver parameters were used for training: 1000 epochs; base learning rate for untrained model, Adam (learning rate = 0.001, beta1 = 0.900, beta2 = 0.999, epsilon = 1.00× 10−8). The role of first model was to detect abnormal signal derived from brain infarction using unsupervised learning. This model consisted of deep convolutional AE3 trained by 5123 normal brain images that train dataset were augmented by horizontally flipping, rotating and jittering brightness, contrast and scale (Figure 1). Second model was Squeeze network4 (SqueezeNet) which diagnosed brain infarction or predicted vascular territory (Figure 2). Raw DWI image, generated image by AE and subtraction image between raw DWI image and generated image, were inputted in the SqueezeNet to diagnose brain infarction, while only raw DWI image was inputted in the network to estimate vascular territory (Figure 3).
RESULTS
The AE trained with only normal brain reproduced the high signal of susceptibility artifact but not the high signal of brain infarction that didn’t exist in normal brain. The accuracy of diagnosing infarct was 0.979 using SqueezeNet where DWI, generated and subtraction images were inputted. Moreover, the accuracy of identifying responsible vascular territory was 0.864 using SqueezeNet where only DWI images were inputted.DISCUSSION
Out deep learning neural network model combining AE and SqueezeNet successfully detected and diagnosed brain infarction, and identified responsible vascular territory automatically from DWI image. AE is a network based on unsupervised learning containing an encoder that converts data to lower dimension and decoder that reconstructs it to the original dimension. Our AE trained with only normal brain images and could restore them including high signals caused by susceptibility artifact that existed in even normal brain (Figure 4). On the other hand, brain infarctions that didn’t exist in normal brain couldn’t be reconstruct well. So, AE generated brain images without infarct, when even abnormal brains which had infarcts were inputted. As a result, only brain infarction appeared by subtracting generated image from raw DWI image. This subtraction image can help SqueezeNet to detect brain infarction. Our brain tissues are sustained from various cerebral arteries. Each brain region that each cerebral artery dominants differs.5 When radiologists assess vascular territory of brain infarction, they predict the occlusion from brain regions where the infarction exists. Deep learning neural network performs well in object recognition including shape, pattern and color.6 Therefore, it can presume responsible vascular territory from brain features. Thus, a deep neural network model combining AE and SqueezeNet that achieved AlexNet7-level accuracy on ImageNet with 50x fewer parameters can be useful to detect exactly brain infarction and protect neuroradiologist from overlooking it under even in an urgent diagnosis. Moreover, the model can be an effective indicator for a determination of the optimal treatment and prediction of the treatment effect.CONCLUSION
A deep learning neural network via AE can detect brain infarction and estimate responsible vascular territory with high accuracy.Acknowledgements
No acknowledgement found.References
- Guberina N, Dietrich U, Radbruch A, et al. Detection of early infarction signs with machine learning-based diagnosis by means of the Alberta Stroke Program Early CT score (ASPECTS) in the clinical routine. Neuroradiology 2018;60(9):889-901.
- Chalela JA, Kidwell CS, Nentwich LM. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet 2007;369(9558):293-8.
- Hinton GE, Salakhutdinov RR. Reducing the dimensionality of data with neural networks. Science 2006;313:504-7.
- Inandola FN, Han S, Moskewicz MW, et al. SqueezeNet: SqueezeNet: AlexNet-level accuracy with 50x fewer parameters and <0.5MB model size. arXiv.org 2018;108:107319111877484.
- Vinken PJ, Bruyn GW. Vascular Disease of the Nervous System, Part 1. North-Holland, Amsterdam 1972;11:24-44.
- Kaiming He, Xiangyu Zhang, Shaoqing Ren, et al. Delving Deep into Rectifiers: Surpassing Human-Level Performance on ImageNet Classification. in 2015 IEEE International Conference on Computer Vision (ICCV), 2015.
- Alex Krizhevsky, Ilya Sutskever, and Geoffrey E. Hinton. ImageNet Classification with Deep Convolutional Neural Networks. In NIPS, 2012.
Figures
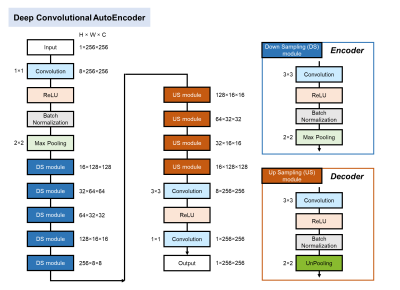
Figure 1. Deep convolutional autoencoder architecture
Our AE consists of the encoder including five down sampling modules (DS module) and the decoder including four up sampling modules (US module). The encoder extracts features from inputted image (1×256×256) and converts the data to lower dimension. On the other hand, the decoder reconstructs compressed data to the original dimension as same as inputted data.
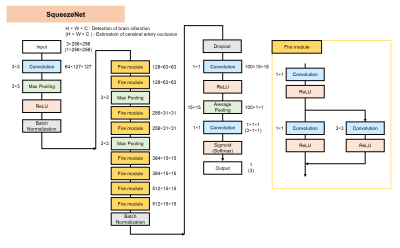
Figure 2. SqueezeNet architecture
SqueezeNet starts with an independent 1×1 Convolution layer, followed by 8 Fire modules and ends with a final 1×1 Convolution layer. The number of filters per Fire module is gradually increased as the layer is deeper. A Fire module is composed of a Squeeze Convolution layer which has only 1×1 Convolution layer and an expand layer which has a combination of 1×1 and 3×3 Convolution layers. Fire module is useful to reduce model size while maintaining the prediction accuracy.
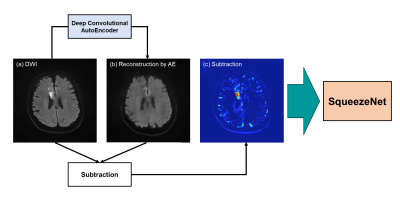
Figure 3. Our deep learning neural network model for the detection and diagnosis of brain infarction and the classification of vascular territory
A raw DWI image (a) was inputted in AE that already trained for normal brain. AE generated a brain image (b) without brain infarction. Additionally, a subtraction image (c) was made by subtracting reconstructed image (b) from a raw DWI image (a). These raw DWI (a), reconstructed (b) and subtraction (c) images were inputted in SqueezeNet to detect brain infarction, while only raw DWI image (a) was inputted to estimate vascular territory.

Figure 4. AE reconstruction that learned only normal brain of DWI (a), (b)
AE which learned only normal brain of DWI could restore not only normal structure (a) but also high signal of susceptibility artifact (b, green arrow). On the other hand, when abnormal brain including brain infarction is inputted, brain infarction isn’t restored (c, green arrow).