1905
Prediction of Hemorrhage Free Survival after Gamma Knife Radiosurgery Based on Preradiosurgical MR Radiomics in Cavernous Malformation1Department of Biomedical Imaging and Radiological Sciences, National Yang-Ming University, Taipei, Taiwan, 2Department of Neurosurgery, Neurological Institute, Taipei Veteran General Hospital, Taipei, Taiwan, 3School of Medicine, National Yang-Ming University, Taipei, Taiwan, 4Brain Research Center, National Yang-Ming University, Taipei, Taiwan, 5Department of Radiology, Taipei Veteran General Hospital, Taipei, Taiwan
Synopsis
Even though the gamma knife radiosurgery (GKRS) shows promising evidence in treating cavernous malformation (CM), there are still a part of patients will have a recurrent hemorrhage after radiosurgery. We proposed a prediction model based on preradiosurgical MR radiomics to estimate the personalized hemorrhage free survival after GKRS. The satisfactory results of the proposed model can benefit the healthcare in patients with CM by providing a reliable prognosis before treatment.
Background and Purpose
Cavernous malformation (CM) is a neurovascular disease that most patients may be asymptomatic in the early stage but with a high-risk occurrence of intracranial hemorrhage, seizures, and headache. In clinical practice, CM is mostly treated by the neurosurgery or gamma knife radiosurgery (GKRS). Compared with the resection surgery, GKRS is a lower risk treatment and more efficient for the lesion located in the deep brain and brainstem with a faster postoperative recovery.1 However, around 20 to 30% patients may still suffer from the recurrent hemorrhage after GKRS.2 In this study, we aim to determine whether the clinical MR characteristics quantified by radiomics analysis before GKRS can be used to predict the hemorrhage free survival (HFS) after radiosurgery in patients with CM.Materials and Methods
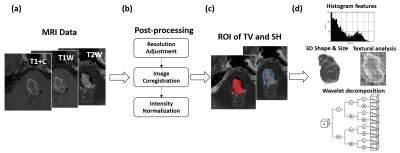
This study was approved by the local Institutional Review Board. A dataset of 212 patients with CM treated by GKRS were retrospectively collected from Taipei Veteran General Hospital. The preradiosurgical MRI data, including postcontrast T1-weighted images (T1+C), T2-weighted images (T2W), and T1-weighted images (T1W), were acquired by using a 1.5-T MR scanner (Signa Horizon LX2, GE Medical Systems) for GKRS treatment planning. Continuous clinical and imaging follow-ups were also collected, and 66 of 212 patients were recorded with hemorrhage recurrence after GKRS (mean of HFS = 33.5 months).Several postprocessing steps on the MRIs were applied to improve the reliability of radiomics analysis. The adjustment of image resolution was first performed to resample all voxel size to 0.50 x 0.50 x 3.00 mm3. The T2W and T1W images were then registered to the subject’s T1+C images followed by the image intensity normalization to transform MR imaging intensity into standardized ranges (Figure 1b). Two sets of lesion ROIs, including the target volume (TV) of CM for GKRS and the surrounding hemosiderin (SH) rim, were delineated by experienced neuroradiologists and researchers (Figure 1c).
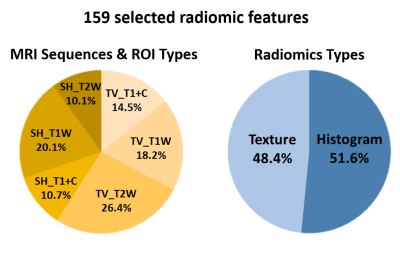
Overall 3526 radiomic features of TV and SH, including histogram, shape/size, and texture features, with wavelet image decomposition were extracted from T1+C, T1W, and T2W images by using the MR Radiomics Platform (Figure 1d).3,4 A machine learning model, Random Survival Forest, was constructed to predict the HFS curve using the MR radiomics as predictors.5 The most informative predictors (159 radiomic features) were selected based on the minimal depth of the decision trees. The patient dataset was than randomly partitioned into two subsets, 80% for training prediction model (170 patients) and 20% for model validation (42 patients). Model performance was evaluated using the time-dependent area under the receiver operator characteristics curves (AUC) with an anticipated value larger than 0.80.
Results and Discussion
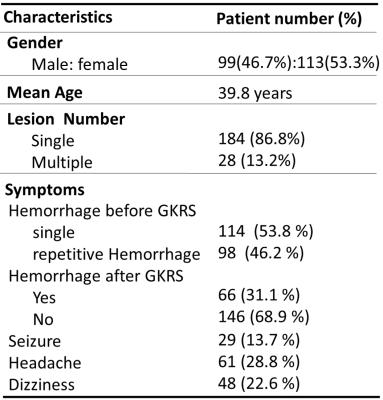
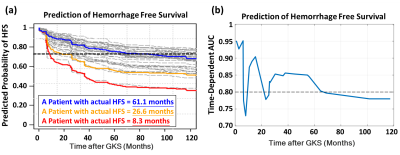
Table 1 lists the clinical characteristics of the recruited 212 patients with CM. The profiles of selected radiomic features showed that both TV- (59.1% of features) and SH-related (40.9% of features) MR radiomics were critical for the HFS prediction (Figure 2). This result suggested that the image traits of SH areas should also be considered which were often ignored in clinical practice. The radiomic types of histogram and texture features (reflecting image inhomogeneity) were the main contributors to the prediction model rather than the size and shape features of the CM (Figure 2). Based on the trained Random Survival Forest model, personalized HFS predictions on the validation dataset can be estimated (gray curves in Figure 3a). The predicted HFS curves for 3 representative patients are in concordance with their actual HFS (color curves in Figure 3a). A higher predicted probability of HFS at a given time point indicated a lower probability for the occurrence of hemorrhage. Furthermore, it was noted that a 0.75 threshold of predicted probability of HFS can be used to predict the potential time point of hemorrhage occurrence. For the 3 representative patients, the predicted time points for hemorrhage based on the 0.75 threshold were 69.9, 25.3, and 9.1 months, while the actual HFS were 61.1, 26.6, and 8.3 months, respectively (Figure 3a). The area under the receiver operating curves (AUC) at different time points after GKRS were between 0.73 and 0.95, suggesting the satisfactory prediction accuracy for the HFS after GKRS based on the preradiosurgical MR radiomics (Figure 3b).Conclusions
The established prediction model based on MR radiomics extracted from both TV and SH regions of CM can satisfactorily predict the HFS after GKRS. This approach can be used to promote the personalized medicine of prognosis and treatment strategy in CM. An independent dataset should be applied to further confirm the efficacy of proposed approach.Acknowledgements
This work was supported by the Ministry of Science and Technology, Taiwan (MOST 106-2221-E-010-016-MY3, MOST 108-2321-B-010-012-MY2).References
1. Wang P, Zhang F, Zhang H, Zhao H. Gamma knife radiosurgery for intracranial cavernous malformations. Clinical neurology and neurosurgery. 2010 Jul 1;112(6):474-7.
2. Shih YH, Pan DH. Management of supratentorial cavernous malformations: craniotomy versus gammaknife radiosurgery. Clinical neurology and neurosurgery. 2005 Feb 1;107(2):108-12.
3. Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, Bussink J, Monshouwer R, Haibe-Kains B, Rietveld D, Hoebers F. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nature communications. 2014 Jun 3;5:4006.
4. Lu CF, Hsu FT, Hsieh KL, Kao YC, Cheng SJ, Hsu JB, Tsai PH, Chen RJ, Huang CC, Yen Y, Chen CY. Machine learning–based radiomics for molecular subtyping of gliomas. Clinical Cancer Research. 2018 Sep 15;24(18):4429-36.
5. Ishwaran H, Kogalur UB, Blackstone EH, Lauer MS. Random survival forests. The annals of applied statistics. 2008;2(3):841-60.
Figures