1899
A Machine Learning Approach for investigation of white matter abnormalities in Parkinson’s disease1radiology, nanjing brain hospital, nanjing, China, 2nanjing brain hospital, nanjing, China, 3Clinical Medicine, Macquarie University, Sydney, Australia, 4Psychiatry, University of Pennsylvania, Philadelphia, PA, United States, 5neurology, nanjing brain hospital, nanjing, China, 6Biomedical Engineering, Peking University, beijing, China
Synopsis
Parkinson's disease (PD) is a common neurodegenerative disorder characterized by disabling motor and non-motor symptoms.1 The abnormalities of white-matter (WM) tracts/regions have been demonstrated in PD. However, previous studies have largely dependent on univariate analysis, such as t-test, which may result in Type-1 error. Further, it remains unclear whether the disruption of WM tracts/regions provided worthwhile information to identify PD from HC. Hence, in current study, a machine learning approach was applied to investigate the white matter profiles of PD.
Introduction
Parkinson’s disease (PD), the second most prevalent neurodegenerative disorder, is accompanied by several motor (including muscular rigidity, bradykinesia, and resting tremor) and non-motor symptoms1. Diffusion tensor imaging (DTI),a noninvasive method to detect, track, and display the white matter tracts in vivo, might help us better to explore the pathogenesis or even discriminate idiopathic Parkinson’s disease (PD) patients from healthy controls (HC).Recently, there has been an increasing interest in DTI in PD which has shed light on our understanding of structural abnormalities underlying PD.Methods
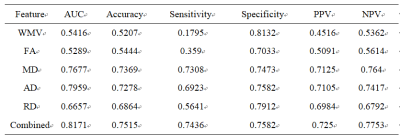
All subjects were collected in Nanjing Brain Hospital using 3T MRI, and the study was approved by the Medical Research Ethical Committee of our hospital. In current study, a total of 78 PD (medical ON) with 91 age-matched healthy controls (HC) were scanned using 3D T1 and DTI sequence. The data preprocessing was using SPM12 and PANDA software,2 thereafter, the white matter volume and diffusion metrics (FA, MD, AD, RD) of the 50 core white matter regions defined in ICBM template were extracted. Last, a machine learning approach using t-test and linear SVM was applied to those white matter features.Results and discussion
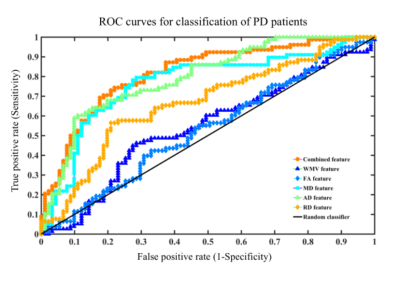
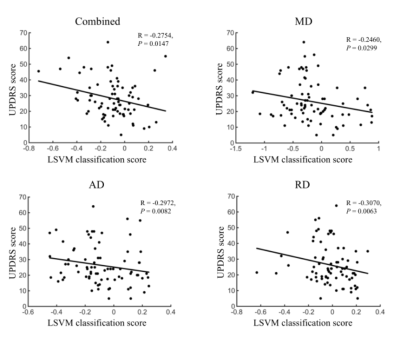
A linear support vector machine (LSVM) classifier achieved an accuracy of 75.15% using these combined white matter features to distinguish PD from HCs. Mean sensitivity was 74.36% and mean specificity was 75.82% (Fig.1 and Table.1). The correlations between UPDRS and LSVM classification scores were significant(Fig.2)(all P<0.05). Notably, the most discriminative features that contributed to the classification were primarily associated with in WM regions with (e.g., fronto-occipital fasciculus, thalamocortical projections, and corpus callosum), the limbic system (e.g., the cingulum, thalamic radiation and fornix), and the motor system (e.g., uncinate fasciculus).Parkinson’s disease (PD) is a heterogeneous multisystem degenerative disorder characterized involved widely regions, involving both motor- and nonmotor-related pathways.3Conclusion
Our results demonstrated a machine learning approach for discriminating PD patients from HC with a good accuracy and sensitivity, it may be useful in a screening setting.Acknowledgements
The authors wish to thank all the participants. In addition, this work was supported by the National Natural Science Foundation of China (81571348, 81701671), National key research and development plan (2016YFC1306600, 2017YFC1310302, 2017YFC1310300), and Jiangsu Natural Science Foundation (BK20151077).Science and Technology Program of Jiangsu Province(BE2019611).References
1. Marti MJ, Tolosa E. Parkinson disease: New guidelines for diagnosis of Parkinson disease. Nature reviews Neurology. Apr 2013;9(4):190-191.
2. Cui Z, Zhong S, Xu P, et al. PANDA: a pipeline toolbox for analyzing brain diffusion images. Frontiers in human neuroscience. 2013;7:42.
3. Devi L, Raghavendran V, Prabhu BM, et al. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. The Journal of biological chemistry. Apr 4 2008;283(14):9089-9100.