1885
A Support Vector Machine Prediction Model for HIV-Status in Adults Using Magnetic Resonance Angiography and Arterial Spin Labeling of the Brain1Physics and Astronomy, University of Rochester, Webster, NY, United States, 2Neurology, University of Rochester, Rochester, NY, United States, 3Imaging Sciences, University of Rochester, Rochester, NY, United States
Synopsis
HIV-infection is known to be related to vascular diseases, which can be explored via cerebral imaging techniques such as magnetic resonance angiography (MRA) and arterial spin labeling (ASL). In this abstract, we use quantitative features extracted from demographic information and vascular imaging data, only, to predict HIV-status in adults using a support vector machine (SVM). This is the first SVM to reasonably predict HIV-status in an aging HIV-population on combination antiretroviral therapy, which may have future biological implications in HIV research.
Introduction
The introduction of combination antiretroviral therapy (cART) has drastically elongated the lifespan of individuals living with HIV. As a result, HIV patients are now able to live to older age, which renders them susceptible to health conditions typically impacting older adults1,2. HIV is a neurodegenerative disease that is also associated with increased vascular risk factors and cardiovascular disease (CVD)3. Magnetic resonance imaging (MRI) provides noninvasive markers of vascular health via arterial spin labeling (ASL) and MR angiography (MRA). The aim of this study was to determine whether quantitative ASL and extracranial vessel (ECV) diameters from MRA could predict HIV-status in older adults.Methods
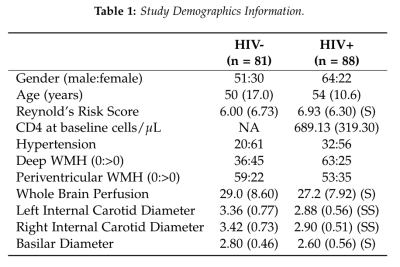
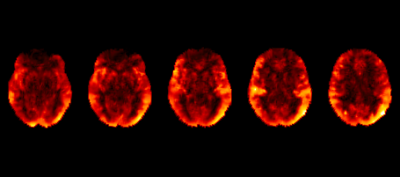
In an ongoing study in an aging HIV population on cART, 169 subjects (mean± SD age = 51.9 ± 14.2 years, range = 19 – 78 years) were evaluated to study the effects of CVD in HIV patients (Table 1). All imaging was conducted on a 3T (Siemens Prisma) scanner equipped with a 64-channel head coil (Erlangen, Germany). The protocol includes high-resolution T1-weighted (T1w) anatomical images using an MPRAGE sequence (TI=950ms, TE/TR=3.87ms/1,620ms, 1mm isotropic resolution). ASL data was collected using a multi-band pseudo-continuous ASL (mb-pCASL) protocol with 5 post-label delays (TE/TR=19ms/3594ms, labeling duration=1.5s, resolution=2.5x2.5x2.3mm3, 90 measurements, EPI factor=86). MRA was acquired using time-of-flight sequences of the neck and brain (TE/TR=3.42ms/21.0ms, GRAPPA=2, 0.5mm isotropic resolution). Cerebral blood flow (CBF) was quantified using oxford_asl4 and region-of-interest (ROI) means were extracted via the Harvard-Oxford atlases in FMRIB’s Software Library (FSL)5 after registration to MNI152 2mm space via the T1w image (Figure 1). ECV diameters were measured by a neuroradiologist. Relationships between variables were assessed and implemented in a support vector classification (SVC) algorithm to predict HIV-status using the scikit-learn module in python6. K-folds validation was performed to assess the accuracy of the algorithm.Results
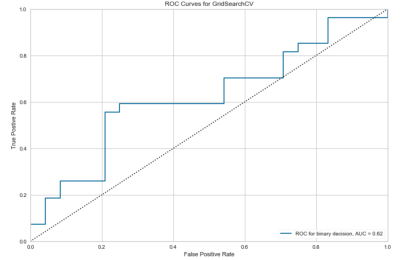
Example CBF maps are shown in figure 1. After hyperparameter tuning via grid search, the optimal SVC uses a radial basis function kernel with penalty and kernel coefficients C=1.5 and gamma=0.01. The no information rate of the population is .521. This algorithm demonstrates K-folds cross-validation accuracy of 68.6%. The receiver operating characteristic area under the curve (ROCAUC) is presented in figure 2.Discussion
We have confirmed that the ECV diameters are significantly smaller in the presence of HIV7, while whole-brain arterial transit time is not different8. One of the implications of this is that the heart must potentially increase cardiac output and blood pressure to meet the oxygen (O2) demands of the brain, leading to increased rates of CVD. Our algorithm demonstrates that it is possible to reasonably predict HIV status in patients without significant carotid atherosclerotic disease with noninvasive vascular imaging, standard blood samples, and medical history without other quantitative imaging or HIV-specific blood biomarkers. While there may not be much current clinical utility in predicting HIV using vascular information, it may be possible in the future to use vascular imaging as a noninvasive way to monitor clinical progress of HIV patients on various cARTs. While we have yet to explore the specific effects of different classes of cARTs, ECV caliber may have the potential to be a biomarker reflective of treatment progress.Conclusion
We have demonstrated that ECV diameters and ROI-based CBF can be used to classify HIV-status in older adults with a higher accuracy than the no information rate. We expect this model to improve upon availability of intracranial vessel diameters and flow pulsatility data. Further, we postulate that ECV caliber and intimal thickness may provide useful clinical markers in monitoring HIV patients on different cARTs.Acknowledgements
This work was made possible by the NIH 5R01AG054328-03 grant. We would also like to acknowledge the study coordinators and study participants.References
1. Berger, A. R., Arezzo, J. C., Schaumburg, H. H., Skowron, G., Merigan, T., Bozzette, S., Richman, D., and Soo, W. 1993. "2',3'-Dideoxycytidine (DdC) Toxic Neuropathy: A Study of 52 Patients." Neurol43358-62.8.
2. Rutschmann, Olivier T., Vernazza, Pietro L., Bucher, Heiner C., Opravil, Milos, Ledergerber, Bruno, Telenti, Amalio, Malinverni, Raffaele, Bernasconi, Enos, Fagard, Catherine, Leduc, Dominique, Perrin, Luc, Hirschel, Bernard, and and the Swiss HIV Cohort Study. 9-29-2000. "Long-Term Hydroxyurea in Combination With Didanosine and Stavudine for the Treatment of HIV-1 Infection. [Miscellaneous Article]." AIDS14(14):2145-51.
3. Vinikoor MJ, Napravnik S, Floris-Moore M, Wilson S, Huang DY, Eron JJ. “Incidence and clinical features of cerebrovascular disease among HIV-infected adults in the Southeastern United States.” AIDS Res Hum Retroviruses. 2013;29(7):1068-74. Epub 2013/04/10. doi: 10.1089/aid.2012.0334. PubMed PMID: 23565888; PubMed Central PMCID: PMCPmc3685694.
4. Chappell, M.A., Groves, A.R., Whitcher, B., Woolrich, M.W. “Variational Bayesian inference for a non-linear forward model.” IEEE Transactions on Signal Processing 57(1):223-236, 2009.
5. Woolrich, M.W., Jbabdi, S., Patenaude, P., Chappell, M., Makni, S., Behrens, T., Beckmann, C., Jenkinson, M., Smith, S.M. “Bayesian analysis of neuroimaging data in FSL.” NeuroImage, 45:S173-86, 2009.
6. Pedregosa et al., “Scikit-learn: Machine Learning in Python.” JMLR 12, pp. 2825-2830, 2011.
7. LaBounty, T. M., Hardy, W. D., Fan, Z., Yumul, R., Li, D., Dharmakumar, R., & Conte, A. H. (2016). “Carotid artery thickness is associated with chronic use of highly active antiretroviral therapy in patients infected with human immunodeficiency virus: A 3.0 Tesla magnetic resonance imaging study.” HIV medicine, 17(7), 516–523. doi:10.1111/hiv.12351.
8. Gutierrez, J., Goldman, J., Dwork, A. J., Elkind, M. S., Marshall, R. S., & Morgello, S. (2015). “Brain arterial remodeling contribution to nonembolic brain infarcts in patients with HIV.” Neurology, 85(13), 1139–1145. doi:10.1212/WNL.0000000000001976.
Figures