1878
Quantitative imaging enhancement with deep learning based denoising: a validation study1Canon Medical Systems Corporation, Tochigi, Japan, 2Canon Medical Systems Europe, Zoetermeer, Netherlands, 3Bordeaux University, Bordeaux, France
Synopsis
In MRI, signal noise ratio is the key point, determining the image quality and its medical relevance. Different ways exist to significantly increase it and then to access to high resolution imaging. Preliminary works introduced deep learning based denoising on several contexts and conclude to a significant signal noise ratio on qualitative images. However, it has not been tested yet on quantitative imaging sequences, questioning its feasibility and potential in this context. In this study, we investigated the DLR impact on calculated T1 and T2 relaxation times and diffusion imaging in healthy human brain areas.
Introduction
In MRI, signal noise ratio (SNR) is the key point, determining the image quality and its medical relevance. Different ways exist to significantly increase it and then to access to high resolution imaging. The most current ones are signal averaging -leading to longer acquisition time- and/or magnetic field increase. More and more, a third way is proposed to drastically improve the SNR without increasing acquisition time: this tool is based on deep learning technology and can be performed retrospectively, after the end of the MR exam. Preliminary works introduced deep learning reconstruction (DLR) on several contexts1,2,3 and conclude to a significant signal noise ratio (SNR) and contrast noise ratio (CNR) gains on qualitative images. However, DLR has not been tested yet on quantitative imaging sequences, questioning its feasibility and potential in this context. In this study, we investigated the putative DLR impact on calculated T1 and T2 relaxation times and diffusion imaging in parallel of the SNR and CNR in healthy human brain areas.Methods
Whole brain explorations have been performed on nine healthy volunteers. They have been scanned on a research 3.0 T MRI scanner (ZGO 100 mT/m, Canon Medical Systems Corporation, Tochigi, Japan) with a 32-channel receiving head coil. The study received IRB approval.Imaging protocol: [1] T1 mapping: 3D MP2RAGE; in-plane resolution=0.7x0.5 mm²; STH=2 mm; TR/TE=7.4 s/3.3 ms; TI=650/3300 ms; inter-shot TR=7 s; NEX=1; TA=3min30. [2] T2 mapping: 2D FSE; in-plane resolution=0.7x0.5 mm²; STH=1.5 mm; TR=5 s; TE=20/60/100/140 ms; NEX=1; TA=2min45. [3] Diffusion Tensor Imaging: 2D SE-EPI; in-plane resolution=1.6 mm3; 30 directions; b values=0-1000 s/mm²; TR/TE=9.2 s/70 ms; NEX=1; TA=5min15.
Data processing: T1, T2 and fractional anisotropy (FA) maps have been generated using Olea Sphere software. Regions of interests (ROIs) have been placed in the frontal white matter (FWM), genu, splenium, thalami and grey matter (GM). SNR and CNR have been estimated on the FSE 1st echo image, selecting ROIs in the WM and the GM. Deep Learning reconstructions have been performed with the application of a deep Convolutional Neuronal Network (dCNN) combined with a low-pass filtered component, in order to maintain original contrasts1.
Results and Discussion
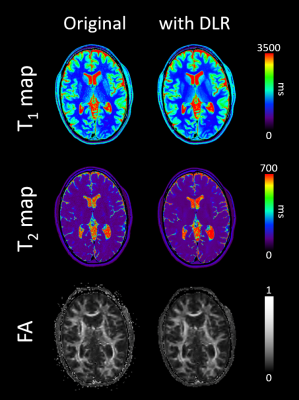
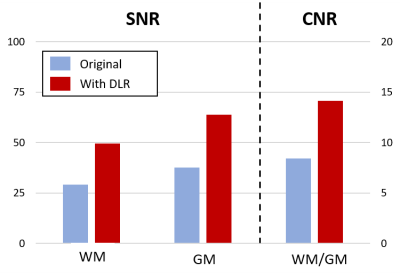
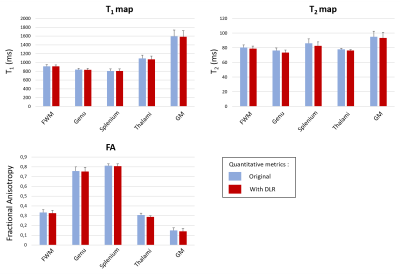
Typical maps of T1, T2 and FA, with and without DLR, are shown in Figure 1. In-plane resolutions and acquisition times have been chosen to generate noisy images (Figure 1, left column). However, DLR images visually drastically improves SNR, allowing a better contrast and delineation between structures (Figure 1, right column). SNR measurements in white and grey matter and CNR between the two structures confirmed a significant gain of 70% after DLR (Figure 2). Mean values of T1, T2 and FA with and without DLR for each region of interest have been plotted in Figure 3. For all these measurements, DLR processing did not change the metrics compared to the original values measured without DLR. These T1, T2 and FA measurements in different brain areas have been found in conformity with literature4,5. However, in addition to preserving the mean, DLR denoising led to a systematic reduction of the standard deviation inside ROIs around 50% for T1 and T2 maps and 10% for FA maps.Conclusion
Following the rising trend of post processing based on artificial intelligence, this work studied the impact of the deep learning denoising on MR quantitative imaging on healthy volunteers. DLR processing allowed to increase the SNR and CNR of brains structures by 70%, without changing the T1, T2 and FA metrics. These preliminary results are very promising and enhance the confidence on the DLR use. Reproducibility has to be tested on different anatomical regions and other sequences, and then a validation on pathological cases is necessary. This new technology can significantly help quantitative imaging protocols to become feasible in the clinic with acquisition times compatible with current practices.Acknowledgements
We would like to thank all the volunteers that contribute to this work.References
1. Kidoh,
Masafumi, Kensuke Shinoda, Mika Kitajima, Kenzo Isogawa, Masahito Nambu,
Hiroyuki Uetani, Kosuke Morita, et al.
Deep Learning Based Noise Reduction for
Brain MR Imaging: Tests on Phantoms and Healthy Volunteers. Magnetic
Resonance in Medical Sciences, 2019.
https://doi.org/10.2463/mrms.mp.2019-0018.
2. Hiroshi Kusahara, Yuki Takai, Kensuke Shinoda, and Yoshimori
Kassai
Evaluation of Variable-TE computed Diffusion Weighted Imaging Technique using
Deep Learning based Noise Reduction. ISMRM Congr. 2019.
3. Ryuichi Mori, Hideki Ota,
Atsuro Masuda, Tomoyoshi Kimura, Tatsuo Nagasaka, Takashi Nishina, Sho Tanaka,
Yoshimori kassai, and Kei Takase
Ultrashort TE Time-Spatial
Labeling Inversion Pulse MR Angiography Denoised with Deep
Learning Reconstruction for
Abdominal Visceral Arteries: A Feasibility Study. ISMRM Congr. 2019.
4. Marques, José P., Tobias Kober, Gunnar
Krueger, Wietske van der Zwaag, Pierre-François Van de Moortele, and Rolf
Gruetter
MP2RAGE, a Self Bias-Field Corrected Sequence for Improved
Segmentation and T1-Mapping at High Field. NeuroImage 49, no. 2
(January 2010): 1271–81. https://doi.org/10.1016/j.neuroimage.2009.10.002.
5. Brander A, Kataja A, Saastamoinen A, Ryymin P, Huhtala H, Ohman J, Soimakallio S, Dastidar P.
Diffusion tensor imaging of the brain in a healthy adult population: Normative
values and measurement reproducibility at 3 T and 1.5 T. Acta Radiol.
2010 Sep;51(7):800-7. doi: 10.3109/02841851.2010.495351.
Figures