1874
Decoupling the default mode network and global state oscillation by neural network-based prediction of the fMRI signal fluctuation1Translational Neuroimaging and Neural Control Group, High Field Magnetic Resonance Department, Max Planck Institute for Biological Cybernetics, Tuebingen, Germany, 2Graduate Training Centre of Neuroscience, International Max Planck Research School, University of Tuebingen, Tuebingen, Germany, 3Danish Research Centre for Magnetic Resonance, Hvidovre, Denmark, 4Howard Hughes Medical Institute, Computational Neurobiology Laboratory, Salk Institute for Biological Studies, La Jolla, CA, United States, 5Division of Biological Sciences, University of California, San Diego, CA, United States, 6Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA, United States
Synopsis
Previously we developed an echo-state network (ESN) to predict the future temporal evolution of the rs-fMRI slow oscillatory feature from both rodent and human brains. In particular, rs-fMRI signals from individual blood vessels that were strongly correlated with neural calcium oscillations were used to train an ESN to predict brain state-specific rs-fMRI signal fluctuations. Here, the ESN-based predictive model was applied to classify rs-fMRI datasets from the Human Connectome Project (HCP). The ESN enables to decouple the brain state-dependent global rs-fMRI signal fluctuation from the intrinsic activity of the default-mode network.
INTRODUCTION
The global rs-fMRI signal is a critical confound of correlation analysis with many contributing factors from both physiological and non-physiological sources. In particular, whether the global mean fMRI signal should be removed before the analysis has been debated1,2. However, recently it has been shown that global fMRI signal fluctuation detected from individual vessels represents changing brain states as both the vascular signal fluctuation and global hemodynamic changes have shown strong correlation with simultaneously acquired neuronal calcium oscillations3,4,5. Importantly, the global signal’s strength is brain state dependent6. Here we focused on the predictability of the global signal as a feature enabling the identification of distinct arousal-mediated brain states.METHODS
Human data acquisitionAll measurements were performed on a 3T Siemens Prisma with a 20-channel receive head coil. 6 healthy adult subjects were scanned using an EPI sequence with the following parameters: TR, 1000 ms; TE, 29 ms; FA. 60°; GRAPPA factor 3; partial Fourier 6/8; matrix, 121 x 119; in-plane resolution, 840 1-1m x 840 IJm; 9 slices with a thickness of 1.5 mm. The duration of each trial of rs-fMRI was 15 minutes with eyes closed.
Training data preprocessing
Locations of single vessels7 penetrating the occipital cortex were found in the mean EPI image. Independent component analysis (ICA)8 was employed for every trial to find a component exhibiting a strong slow fluctuation and its corresponding spatial map highlighting the dominating vascular contribution. The ICA map and the mean EPI image combined allowed to extract time courses only from venules displaying strong slow fluctuations. After normalizing the data, the signals were bandpass filtered in the 0.01-0.1 Hz frequency range to extract the slowly changing feature. To create the training dataset, normalized raw signals were coupled with the slow oscillatory signals shifted by 10 s to enforce future prediction.
Human Connectome Project (HCP) data
3279 rs-fMRI sessions of the HCP9 dataset were used. DMN and single hemisphere V1 signals were extracted based on atlases10,11. The DMN-ICA spatial map and corresponding time courses were taken from the 15 component ICA decomposition provided by the HCP. All signals were resampled to match the 1 s TR of the in-house dataset.
Echo state network (ESN) prediction
The ESN12,13 was trained to predict the slow oscillatory feature of raw input signals 10 s ahead. To do this, at every time step, the network encoded input temporal patterns 𝑥(𝑡) into a hidden state vector 𝒉(𝑡) based on the equation:
𝒉(𝑡) = (1 − 𝑎𝛾)𝒉(𝑡 − 1) + 𝛾𝑓 (𝑾𝑖𝑛𝑥(𝑡) + 𝑾𝒉(𝑡 − 1) + 𝑾𝑓𝑏𝑦(𝑡 − 1)),
where 𝑾𝑖𝑛, 𝑾, 𝑾𝑓𝑏 were the input, internal and feedback connectivity matrices, 𝑓 was the hyperbolic tangent function and 𝑎, 𝛾 were hyperparameters influencing the network’s memory property. The output prediction 𝑦 was generated by a linear weighting of the hidden state vector:
𝑦(𝑡) = 𝒘𝑜𝑢𝑡𝒉(𝑡)
Output weights were set with supervision. Hyperparameter values were found using random search14 and cross validation.
RESULTS
Previously we reported training an ESN to predict the temporal evolution of the slow fluctuation of human vasculature based on single-vessel rs-fMRI data15 (Fig.1). Here we applied the trained network on data coming from the HCP. We predicted V1 signals (n=6558) as our training data were extracted from vessels penetrating the occipital cortex (Fig.2AB). To examine signal features driving the predictions, we focused on the 5% of best and worst predicted sessions (Fig.2B). Much stronger slow fluctuations were observed in the well predicted signals (Fig.2C). To investigate brain states influencing prediction we computed seed-based whole-brain correlation maps using the predicted V1 signals as seeds (Fig.3). Fig.4AB shows the difference of mean correlation maps between the well and poorly predicted groups. Due to the global increase of correlation in the well predicted group, in the following analysis we used global cortical signals as seeds. The resulting map looks very similar to the V1 map (Fig.4CD) suggesting that in the well predicted trials, the V1 signal was dominated by the global activity. We repeated the analysis using average DMN signals as seeds. Interestingly, internal DMN correlations didn’t show significant differences despite a global increase in synchrony with the rest of the cortex (Fig.4EF). A possible explanation of this was that in sessions dominated by the global signal, the internal DMN connectivity was reduced. To test this possibility, we used DMN-ICA signals as seeds (Fig.5D). Due to the nature of ICA these signals were less affected by the global component and represented intrinsic DMN activity. The resulting map shows reduced internal DMN connectivity in global signal dominated sessions (Fig.5EF).DISCUSSION & CONCLUSION
The influence of the presence and strength of the global signal on rs-fMRI signal prediction, suggests that brain activity patterns of less aroused6,16,17 subjects are more predictable based on the trained neural network, but the strength of their internal DMN correlations is reduced. The decoupling of intrinsic networks and the global signal has previously been reported in anesthetized monkeys18, here we report it in awake human subjects using non-invasive methods. A promising direction for future research is extending the predictive system to EEG and whole-brain fMRI datasets to specify brain dynamics in different states across multiple scales in both normal and diseased subjects.Acknowledgements
This research was supported by NIH Brain Initiative funding (RF1NS113278-01), and the S10 instrument grant (S10 RR023009-01) to Martinos Center, German Research Foundation (DFG) Yu215/3-1, BMBF 01GQ1702, and the internal funding from Max Planck Society. We thank Dr. R. Pohmann and Dr. K. Buckenmaier for technical support; Dr. E. Weiler, Dr. P. Douay, Mrs. R. König, Ms. S. Fischer, and Ms. H. Schulz for animal/lab maintenance and support; the Analysis of Functional NeuroImages (AFNI) team for software support.References
1. Murphy, K., Birn, R.M., Handwerker, D.A., Jones, T.B., and Bandettini, P.A. (2009). The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage 44,893-905.
2. Fox, M.D., Zhang, D., Snyder, A.Z., and Raichle, M.E. (2009). The global signal and observed anticorrelated resting state brain networks. J Neurophysiol 101, 3270-3283.
3. He, Y., Wang, M., Chen, X., Pohmann, R., Polimeni, J.R., Scheffler, K., Rosen, B.R., Kleinfeld, D., and Yu, X.(2018). Ultra-Slow Single-Vessel BOLD and CBV-Based fMRI Spatiotemporal Dynamics and Their Correlation with Neuronal Intracellular Calcium Signals. Neuron 97, 925-939 e925.
4. Du, C., Volkow, N.D., Koretsky, A.P., and Pan, Y. (2014). Low-frequency calcium oscillations accompany deoxyhemoglobin oscillations in rat somatosensory cortex. Proceedings of the National Academy of Sciences of the United States of America 111, E4677-4686.
5. Ma, Y., Shaik, M.A., Kozberg, M.G., Kim, S.H., Portes, J.P., Timerman, D., and Hillman, E.M. (2016).Resting-state hemodynamics are spatiotemporally coupled to synchronized and symmetric neural activity in excitatory neurons. Proceedings of the National Academy of Sciences of the United States of America 113, E8463-E8471.
6. Fukunaga, M., Horovitz, S.G., van Gelderen, P., de Zwart, J.A., Jansma, J.M., Ikonomidou, V.N., Chu, R., Deckers, R.H.R., Leopold, D.A., Duyn, J.H., 2006. Large-amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and early sleep stages. Magnetic Resonance Imaging 24, 979–992.
7. Yu, X., He, Y., Wang, M., Merkle, H., Dodd, S.J., Silva, A.C., and Koretsky, A.P. (2016). Sensory and optogenetically driven single-vessel fMRI. Nat Methods 13, 337-340.
8. Bell, A.J., and Sejnowski, T.J. (1995). An information-maximization approach to blind separation and blind deconvolution. Neural Comput 7, 1129-1159.
9. Van Essen, D.C., Ugurbil, K., Auerbach, E., Barch, D., Behrens, T.E., Bucholz, R., Chang, A., Chen, L., Corbetta, M., Curtiss, S.W., et al. (2012). The Human Connectome Project: a data acquisition perspective. Neuroimage 62, 2222-2231.
10. Glasser, M.F., Coalson, T.S., Robinson, E.C., Hacker, C.D., Harwell, J., Yacoub, E., Ugurbil, K., Andersson, J., Beckmann, C.F., Jenkinson, M., et al. (2016). A multi-modal parcellation of human cerebral cortex. Nature 536, 171-178.
11. Yeo, B.T., Krienen, F.M., Sepulcre, J., Sabuncu, M.R., Lashkari, D., Hollinshead, M., Roffman, J.L., Smoller, J.W., Zollei, L., Polimeni, J.R., et al. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106, 1125-1165.
12. Jaeger, H. (2001). The "echo state" approach to analysing and training recurrent neural networks.
13. Jaeger, H., Lukosevicius, M., Popovici, D., and Siewert, U. (2007). Optimization and applications of echo state networks with leaky-integrator neurons. Neural Netw 20, 335-352.
14. Bergstra, J., and Bengio, Y. (2012). Random search for hyper-parameter optimization. J Mach Learn Res13, 281-305.
15. Sobczak, F.; He, Y.; Yu, X.: Predict the slow oscillation of the single-vessel resting-state fMRI signal of rats and humans with echo state networks. In Joint Annual Meeting ISMRM-ESMRMB 2018, 0436. Joint Annual Meeting ISMRM-ESMRMB 2018, Paris, France. (2018)
16. Liu, X., de Zwart, J.A., Scholvinck, M.L., Chang, C., Ye, F.Q., Leopold, D.A., and Duyn, J.H. (2018).Subcortical evidence for a contribution of arousal to fMRI studies of brain activity. Nat Commun 9, 395.
17. Wong, C.W., Olafsson, V., Tal, O., Liu, T.T., 2013. The amplitude of the resting-state fMRI global signal is related to EEG vigilance measures. NeuroImage 83, 983–990.
18. Turchi, J., Chang, C., Ye, F.Q., Russ, B.E., Yu, D.K., Cortes, C.R., Monosov, I.E., Duyn, J.H., and Leopold,D.A. (2018). The Basal Forebrain Regulates Global Resting-State fMRI Fluctuations. Neuron 97, 940-952.e944.
Figures
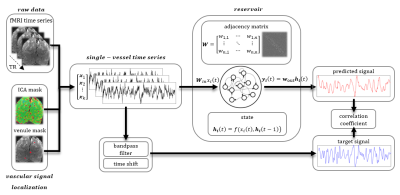
Fig. 1. Prediction system operation pipeline
Raw vascular data are extracted from fMRI data using venule and ICA masks. These temporal signals are inputs to the ESN. They are also bandpass filtered and shifted by 10 seconds to become target outputs of the network.The reservoir encodes the temporal dynamics of input signals into state vectors. The decoder interprets these states and generates a prediction of the slow fluctuation’s value 10 s ahead. After generating the full predicted time series the prediction is compared with the target output using Pearson’s correlation coefficient.
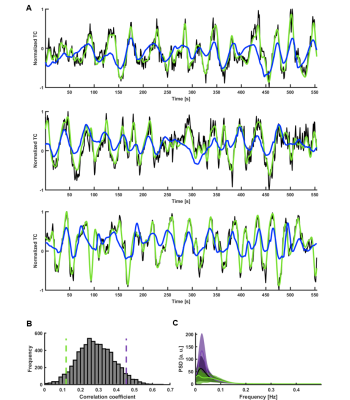
Fig. 2. ESN categorization of V1 temporal patterns
A. Predictions of signals with the three best correlations (CC1=0.65, tlag,1=-1s; CC2=0.61, tlag2=2s; CC3=0.61, tlag3=2s; black – raw data, green – target, blue – network prediction).
B. Histogram of prediction scores obtained by predicting slow fluctuations of 6558 single-hemisphere V1 ROI signals extracted from HCP data. Green and violet dashed lines mark the bottom and top 5% of correlation coefficients.
C. Mean PSDs of time courses whose predictions obtained the bottom 5% (green) and top 5% (violet) scores. Shaded areas show SD.
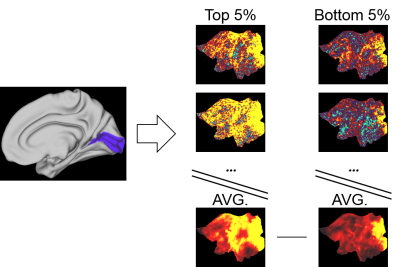
Fig. 3. Seed-based correlation group comparison
A seed region mask (here V1 marked in blue) was used to extract time series from each of the 5% best and worst predicted trials. These signals were then correlated with all other brain voxels to create seed-based correlation maps. The maps were averaged across groups and subtracted to create maps displaying correlation differences between the well and poorly predicted groups.
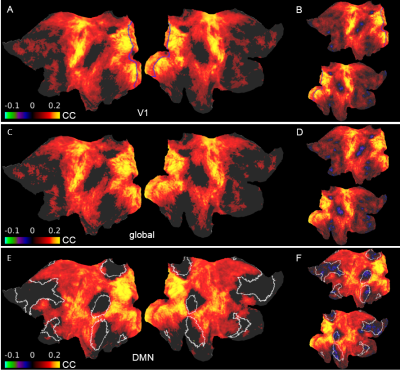
Fig. 4. Difference maps of seed-based correlations between well and poorly predicted sessions
A. V1 signals were the seeds (blue). Visual, sensorimotor and auditory areas display high increases in correlation. C. Average cortical signals were the seeds. The resemblance to the V1 result suggests that V1 signals of the “top” group were driven by the global signal. E. DMN signals were the seeds (white). Despite showing increased synchrony with most of the cortex, ROIs constituting the DMN don’t show significant differences. BDF. Same as ACE but insignificant differences aren’t masked.
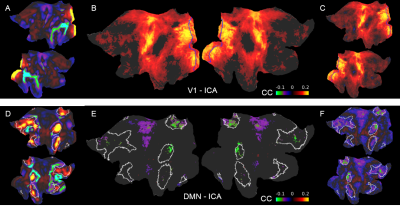
Fig. 5. Difference maps of ICA seed-based correlation between well and poorly predicted sessions
A. V1 ICA component spatial map. B. Difference between the mean V1 ICA seed-based correlation maps of the two groups. The result resembles the pattern obtained by using V1 ROI seeds. D. DMN ICA component spatial map. DMN ROIs are marked by white borders. E. Difference between the mean DMN ICA seed-based correlation maps of the two groups. The intrinsic DMN ICA signals show significantly reduced connectivity with DMN areas. CF. Same as BE but insignificant differences aren’t masked.