1860
CDP-choline may influence metabolite levels in the anterior cingulate of people in remission of depression1Institute of Mental Health Research, Ottawa, ON, Canada, 2Cellular and Molecular Medicine, University of Ottawa, Ottawa, ON, Canada, 3The Ottawa Hospital, Ottawa, ON, Canada, 4Radiology, University of Ottawa, Ottawa, ON, Canada
Synopsis
Cognitive deficits often persist beyond remission in major depressive disorder (MDD). CDP-choline has shown improvements in cognition in other populations characterized by cognitive deficits. These improvements may be due to interactions with the glutamatergic and cholinergic systems. The aim of this ongoing study was to assess the neurochemical response through magnetic resonance spectroscopy to the acute administration of CDP-choline compared with placebo in the anterior cingulate in a group of remitted MDD participants. Early results indicate possible relationships between glutamate and myo-inositol concentrations, and CDP-choline.
Introduction
Major depressive disorder (MDD) is a debilitating condition characterized by prolonged and debilitating sadness or anhedonia. Often, these symptoms are also accompanied by functional and cognitive deficits1. Medications that target the serotonergic system (i.e. the most typical medication class for treating depression) exert some benefit in targeting these latter deficits, but effects are inconsistent2. Crucially, cognitive deficits tend to persist following depression remission, particularly, in the context of multiple depressive episodes. Based on work in other populations characterized by cognitive deficits, drugs that target the glutamatergic and cholinergic systems can have pro-cognitive effects. The endogenous agonist of nicotinic receptors, choline, appears to increase glutamate release3 and has been shown to improve cognition4. Choline can be ingested as a dietary supplement as CDP-choline4. As such, the aim of this study was to assess the neurochemical response through magnetic resonance spectroscopy (MRS) to the acute administration of CDP-choline compared with placebo in a group of remitted MDD (MDDR) participants. Comparisons with healthy controls (HC), with no history of depression or cognitive deficits were also carried out.Methods
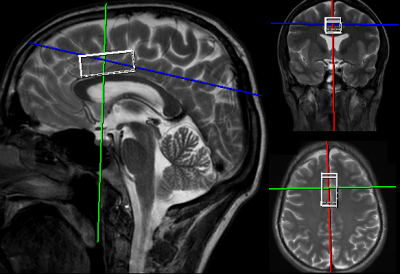
Thirteen patients in remission from depression (29.5 +/- 7.6 years) and 11 healthy controls (28.9 +/- 9.5 years) provided informed written consent to participate in the study. Each participant in the MDDR group was scanned twice on a 3T Siemens Biograph mMR (Siemens, Erlangen) on two visits separated by ≥1 week. The MDDR participants were given 2000 mg of either CDP-choline or placebo (double-blind) 4 hours before beginning the scanning protocol on the first visit; the other substance was administered on the next visit. A water-suppressed PRESS sequence was acquired (TE=30 ms, TR=2000 ms, averages=128) from a 2 x 4 x 1.5 cm3 voxel placed in the bilateral dorsal anterior cingulate cortex, which is a region implicated in MDD and cognition (Figure 1). Excitation and water suppression flip angles were optimized for each participant, and shimming was performed using FASTESTMAP5. An additional 8 average water-unsuppressed PRESS sequence was acquired with the same parameters for eddy-current correction and for determining metabolite concentrations. All fitting was done using LCModel6. Glutamate + glutamine (Glx), Cr, NAA, choline, and myo-Inositol (Myo) concentrations were examined after CDP-choline and placebo administration in the MDDR group and between the HC and MDDR groups. Only metabolites with CRLB < 20% were included in the analyses.Results
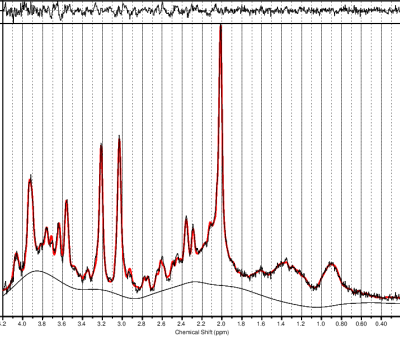
High-quality spectra were acquired, with very little residual observed from the fit (Figure 2). CRLB of Glx, Cr, NAA, choline, and Myo were all well below 20%. No significant differences were observed in any metabolites between the HC and MDDR group after ingesting either CDP-choline or placebo. Trend-level increases were observed in the MDDR group for Glx (p=0.070) and Myo (p=0.056) concentrations after taking CPD-choline versus placebo. No differences were observed in Cr, NAA, or, surprisingly, choline.Discussion
Previous work has shown that MDD is associated with reduced glutamate concentrations relative to healthy controls7, but it has also been shown that these levels can recover in remission8. Similarily, in this study, Glx concentrations were not significantly lower in the MDDR compared with the HC group. The trend-level difference in Glx after taking CPD-choline is consistent with the observation that choline can upregulate glutamate levels.There are several studies that have shown a reduction in Myo in MDD compared to healthy controls7,9,10. It is possible that CPD-choline may improve cognition through actions that increase Myo. Surprisingly, no changes in neuronal choline concentrations after administration of CPD-choline were observed. It may be that the induced differences in choline concentrations are simply not large enough to be reliably detected via MRS.
This study is ongoing; thus, it is possible that the trend-level differences observed will reach significance. We also expect to correlate these MRS measurements with cognitive performance and daily functioning.
Conclusion
Though no significant differences were observed in these interim analyses, it appears that CPD-choline may have an influence on glutamate and Myo levels in the anterior cingulate cortex of people in remission from depression. Such insight has significance with respect to treating lingering cognitive deficits associated with depression.Acknowledgements
This work was funded through the University Medical Research Fund (UMRF).References
1. McIntyre R, Cha D, Soczynska J, et al. Cognitive deficits and functional outcomes in major depressive disorder: determinants, substrates, and treatment interventions. Depress Anxiety. 2013;30(6):515-527.
2. Bortolato B, Carvalho A, McIntyre R. Cognitive Dysfunction in Major Depressive Disorder: A State-of-the-Art Clinical Review. CNS Neurol Disord - Drug Targets. 2015;13(10):1804-1818. doi:10.2174/1871527313666141130203823
3. Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383(6602):713-716. doi:10.1038/383713a0
4. Gareri P, Cotroneo AM, Castagna A, et al. Effectiveness and safety of citicoline in mild vascular cognitive impairment: the IDEALE study. Clin Interv Aging. February 2013:131. doi:10.2147/CIA.S38420
5. Gruetter R, Tkáč I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med. 2000;43(2):319-323. doi:10.1002/(SICI)1522-2594(200002)43:2<319::AID-MRM22>3.0.CO;2-1
6. Provencher S. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672-679.
7. Yildiz-Yesiloglu A, Ankerst DP. Review of 1H magnetic resonance spectroscopy findings in major depressive disorder: A meta-analysis. Psychiatry Res - Neuroimaging. 2006;147(1):1-25. doi:10.1016/j.pscychresns.2005.12.004
8. Järnum H, Eskildsen SF, Steffensen EG, et al. Longitudinal MRI study of cortical thickness, perfusion, and metabolite levels in major depressive disorder. Acta Psychiatr Scand. 2011;124(6):435-446. doi:10.1111/j.1600-0447.2011.01766.x
9. Taylor R, Osuch EA, Schaefer B, et al. Neurometabolic abnormalities in schizophrenia and depression observed with magnetic resonance spectroscopy at 7 T. BJPsych Open. 2017;3(1):6-11. doi:10.1192/bjpo.bp.116.003756
10. Coupland NJ, Ogilvie CJ, Hegadoren KM, Seres P, Hanstock CC, Allen PS. Decreased prefrontal myo-inositol in major depressive disorder. Biol Psychiatry. 2005;57(12):1526-1534. doi:10.1016/j.biopsych.2005.02.027
Figures