1856
Neuronal and Astroglial Metabolism in icv-STZ Mouse Model of Alzheimer’s Disease: A 1H-[13C]-NMR Study1NMR spectroscopy and Microimaging, CSIR-CCMB, Hyderabad, India, 2NMR Spectroscopy and Microimaging, CSIR-CCMB, Hyderabad, India
Synopsis
Alzheimer’s Disease (AD) has been categorized into familial (~5%) and sporadic AD (~95%). Familial cases are genetic whereas sporadic AD (sAD) is acquired during individual’s lifespan. Intracerebroventricular(icv) streptozotocin(stz) administered animals has been shown to resemble AD phenotype through many molecular studies. In this study we have investigated the impact of icv-stz treatment on memory and neurometabolic activity by infusion of [1,6-13C2]Glucose and [2-13C]Acetate in conjunction with NMR spectroscopy. Our findings have shown the compromised memory, perturbed neurometabolic homeostasis along with decreased synaptic neurotransmission across glutamatergic and GABAergic synapse in cortical and hippocampal brain regions of icv-stz treated mouse.
INTRODUCTION
Alzheimer’s disease (AD) is the most common neurodegenerative disorder associated with gradual deterioration in memory and cognitive functions. The disease commonly exists in two forms, familial (fAD) and sporadic (sAD), based upon its origin1. The familial AD, though rare (5%), has been extensively studied due to availability of many transgenic animal models. In contrast, sporadic AD which imparts around 95% of total is not so well investigated. Intracerebroventricular (icv) administration of streptozotocin (STZ) in mouse leads to death of neurons and other cells, and is believed to represent sAD. Several molecular studies have shown the resemblance of (icv-STZ) model to AD patients. However, the impact of icv-STZ administration on neuronal and astroglial function is lacking. The present study assessed the neuronal and astroglial metabolic activity in the cerebral cortex and hippocampus of icv-STZ mouse model of AD by 1H-[13C]-NMR spectroscopy in conjunction with an infusion of [1,6-13C2]Glucose and [2-13C]Acetate, respectively2MATERIALS AND METHODS
All animal experiments were performed under approved protocols by the Institutional Animal Ethics Committee of CCMB. Male C57BL6 (6 month old) mice were divided into two groups: icv-STZ (n=15) and SHAM (n=15). Mice were anaesthetized using ketamine:xylazine mixture (100:10 mg/Kg, ip). The heads were shaved and fixed in the Stoelting stereotax. STZ (dissolved in aCSF:citrate buffer) was administered (5 mg/kg, ICV) in ventricles by infusing 2 ul in 5 min3, and scalp was stitched. Memory and brain energy metabolism of mice were assessed 2 months after STZ administration. Memory was assessed by Novel Object Recognition Test (NORT)4. For measurement of neurometabolic activity, animals were kept on fasting for 3 hrs. [1,6-13C2]Glucose or [2-13C]Acetate were administered in awake mice trough tail vein for 2 min. Blood was collected from retro orbital sinus followed by fixing the brain metabolites using focused beam microwave irradiation (4kw for 1 s) after 7 minutes of [1,6-13C2]Glucose or 10 min for [2-13C]Acetate administration. The cerebral cortex and hippocampus were dissected and stored in liq. N2. Metabolites were extracted from frozen tissue. The concentration and 13C labeling of amino acids were measured in 1H-[13C]-NMR spectra recorded at 600 MHz Bruker Avance HD III NMR spectrometer. The cerebral metabolic rates of glucose oxidation (CMRGlc) by glutamatergic, GABAergic neurons and astroglia were determined from the 13C labeling of brain amino acids from [1,6-13C2]glucose and [2-13C]acetate. Student T test was carried out to understand the statistical significance of difference of measurement in STZ treated and control mice. All the data are presented as Mean±SEM.RESULTS
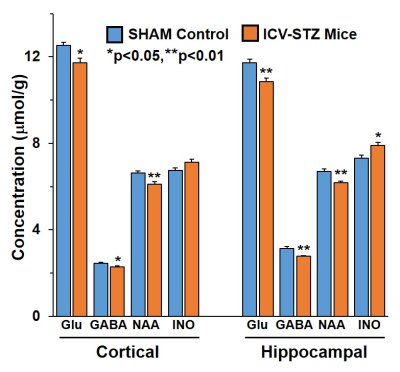
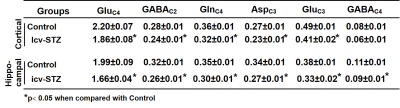
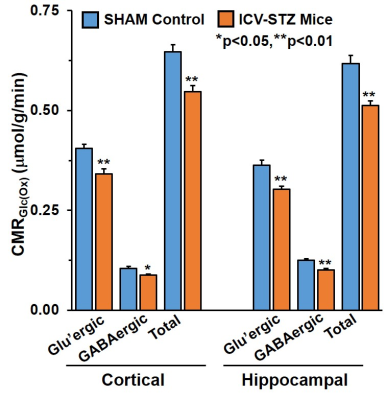
The memory analysis using NORT indicates icv-STZ treated mice (28.5±4.6%) spent significantly (p=0.0001) less time with novel object as compared to SHAM operated controls (68.4±4.4%) suggesting memory impairment in these animals. The levels of Glutamate (12.5±0.1 vs 11.7±0.2 mmol/g, p=0.01), GABA (2.4±0.05 vs 2.3±0.03 mmol/g, p=0.02) and NAA (6.6±0.1 vs 6.1±0.1mmol/g, p=0.002) were decreased in the cerebral cortex of icv-STZ treated mice when compared with (Fig. 1). Similar results were seen in the hippocampus (Fig 1). Additionally, there was an increase in the level of inositol (7.3±0.1 vs 7.8±0.2 mmol/g, p=0.01) in the hippocampus. There was no significant change in 13C labeling of amino acids from [2-13C]acetate in STZ-treated mice suggesting STZ treatment have no impact on astroglial metabolic activity in the cerebral cortex (SHAM 0.1±0.01; icv-STZ 0.1±0.01mmol/g/min, p=.0.51) and hippocampus (SHAM 0.1±0.01; icv-STZ :0.1±0.01 mmol/g, p=.0.54). The concentrations of labeled GluC4, GABAC2, GlnC4, AspC3 and GluC3 from [1,6-13C2]Glucose were decreased significantly (p<0.01) in the cerebral cortex as well as in the hippocampus of icv-STZ mice when compared with SHAM controls (Table 1). Consequently, the CMRGlc associated with glutamatergic and GABAergic neurons was reduced (p<0.001) in the cerebral cortex and hippocampus of icv-STZ treated mice (Fig. 2).DISCUSSION
These data suggest streptozotocin administration in ventricles compromised memory, neurometabolic homeostasis, and synaptic transmission associated with glutamatergic and GABAergic neurons in the cerebral cortex and hippocampus. As these parameters are very well-established markers of AD5, a detailed investigation of icv-STZ model will provide a better insight of the mechanism involved in the pathology of sporadic AD. This may be useful for the development of therapeutics for sAD.Acknowledgements
Authors acknowledge the financial support from CSIR-CCMB to conduct this study.References
1. Selkoe DJ (1989) Amyloid beta protein precursor and the pathogenesis of Alzheimer's disease. Cell 58:611.
2. Mishra et al (2018) Subanesthetic ketamine reverses neuronal and astroglial metabolic activity deficits in a social defeat model of depression. J Neurochem 146(6):722-734.
3. Hoyer S et al (1998) Is sporadic Alzheimer disease the brain type of non-insulin dependent diabetes mellitus? A challenging hypothesis. J Neural Transm 105(4-5):415.
4. Lueptow LM1 (2017) Novel Object Recognition Test for the Investigation of Learning and Memory in Mice J Vis Exp. 126
5. Tiwari V and Patel AB (2012) Impaired glutamatergic and GABAergic function at early age in AβPPswe-PS1dE9 mice: implications for Alzheimer's disease. J Alzheimers’s Dis 28:765