1853
Brain and immune system crosstalk: Examining the link between proinflammatory markers and glial markers measured with 1H-MRS at 7T1Danish Research Centre for Magnetic Resonance, Centre for Functional and Diagnostic Imaging and Research, Copenhagen University Hospital, Hvidovre, Denmark, 2Department of Radiation Sciences, Umeå University, Umeå, Sweden, 3Institute of Sports Medicine Copenhagen (ISMC), Copenhagen University Hospital Bispebjerg, Copenhagen, Denmark, 4Center for Magnetic Resonance, Department of Health Technology, Technical University of Denmark, Lyngby, Denmark, 5Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark, 6Department of Neurology, Copenhagen University Hospital Bispebjerg, Copenhagen, Denmark
Synopsis
Inflammation is proposed as one of the central pillars driving ageing. This study was the first to link peripheral inflammation to brain metabolite differences during normal ageing and to associate this to visuo-spatial working memory performance. Our data shows how peripheral inflammatory markers were associated with performance on visuo-spatial working memory and glial metabolic markers in ACC, Hippocampus, and Thalamus. The findings highlight the role of glial cells as part of the neurobiological link between peripheral inflammation and cognitive performance during ageing.
Introduction
Inflammation is proposed as one of the central pillars driving ageing1. Inflammatory processes are related with alterations in brain cellular metabolism. The activation of brain immune cells, such as astrocytes and microglia, is one of the essential actions in neuroinflammation and is hypothesized to cause neurodegeneration and cognitive decline2. A link between peripheral and central inflammation is supported by the extensive crosstalk between the brain and the immune system, but the neurobiological mechanism linking it with cognitive performance differences in aging is missing. In this study we examined the relationship between pro-inflammatory markers, glial metabolic markers, and cognitive performance across the lifespan.Methods
ParticipantsSixty healthy participants were recruited and equally distributed into three age groups: younger (18-26 years old), middle (39-50 years old) and older (69-79 years old). We included an equal distribution of men and women.
Magnetic resonance
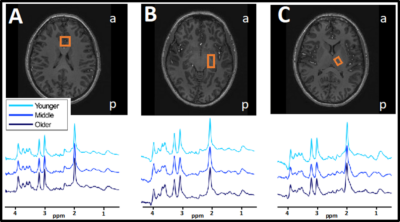
Semi-localized by adiabatic selective refocusing (sLASER) 1H-MRS data3,4 (TR/TE=3700/32 ms, bandwidth=4 kHz, data points=2048) was collected with a Philips 7T whole body MR scanner (Philips, Best, Netherlands) equipped with a dual transmit coil and a 32-channel receive head coil (Nova Medical, Wilmington, MA, USA). Voxels were placed in the medial ACC (20x20x20 mm3, 16 acquisitions), left hippocampus (30x15x15 mm3, 64 acquisitions) and left thalamus (16x12x16 mm3, 64 acquisitions). At the beginning of each scan, a non-water suppressed spectrum was acquired and afterwards variable pulse power and optimized relaxation (VAPOR) delay water suppression5 was applied. The FASTERMAP algorithm was used for second order B0 shimming for each voxel separately with the shim box 15 mm larger in each direction than the voxel and centered on the voxel. Spectra were fitted with LCModel using a basis set with 20 metabolites and a measured macromolecular baseline. Estimates of metabolite concentration were corrected for tissue fractions in the voxel including tissue specific attenuation factors for T1 and T2 relaxation times. Levels of myo-inositol (mINS), total Choline (tCho, GPC+Pch) were used for the main analyses.
Proinflammatory markers
Blood was drawn from each participant. EDTA-plasma was separated by centrifugation, aliquoted, and stored at -80 °C within two hours. Plasma levels of TNF-α and IL-8 were measured in duplicate analyses using enhanced sensitivity bead arrays Human High Sensitivity Cytokine Premixed Kit A (R&D Systems, Minneapolis, MN). High-sensitivity CRP was determined in duplicates using a quantitative sandwich immunoassay technique (R&D Systems, Minneapolis, MN).
Cognitive testing
Cambridge Neuropsychological Test Automated Battery (CANTAB) was used to assess visuospatial working memory (vsWM), which was derived as a composite of two tests, paired associates learning, and spatial working memory (Cambridge Cognition Ltd., Cambridge, United Kingdom).
Statistics
SPSS 25 (SPSS, Chicago, IL, USA) was used for statistical analysis. Spearman’s rank correlations were calculated to estimate the association between inflammatory markers, metabolite concentrations, and cognitive performance respectively. Significant statistical threshold was determined as p < 0.05, and Bonferroni correction was applied to account for multiple testing.
Results
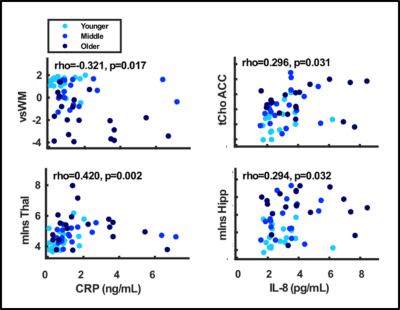
There was a significant difference across all three age-groups in CRP and IL-8, but not for TNF-a. There was a significant negative correlation between CRP and performance on vsWM (rho=-0.321, p=0.017), and a significant positive correlation with mIns in thalamus (rho=0.420, p=0.002). There was a significant positive correlation between IL-8 and tCho in ACC (rho=0.0296, p=0.031) and mIns in hippocampus (rho=0.294, p=0.032).Discussion
Concentrations of the proinflammatory CRP and IL-8 were higher in the older than the younger group, indicative of inflammation. These peripheral inflammatory markers were associated with performance on visuo-spatial working memory as well as glial metabolic markers in ACC, hippocampus, and thalamus. This study adds to the literature linking peripheral inflammation and cognitive impairment to neurobiological brain changes during ageing. In older individuals, peripheral inflammation has previously been associated with brain atrophy, white matter microstructural disintegration and hyperintensities, lower regional cerebral blood flow and altered functional connectivity6,7,8,9, now we add brain metabolites and in particular glial markers. This study further substantiates the importance of thalamus hippocampus, ACC for the age-related associations with cognitive performance.Conclusion
Peripheral inflammation was associated with performance on visuospatial working memory as well as glia-related brain metabolites. Glial cells may, thus, be part of the neurobiological link between peripheral inflammation and cognitive performance during ageing.Acknowledgements
The 7T scanner was donated by the Danish Agency for Science, Technology and Innovation grant no. 0601-01370B, and The John and Birthe Meyer Foundation.References
1. Kennedy B K, Berger S L, Brunet A, et al. Geroscience: Linking aging to chronic disease. Cell 2014; 159, 709–713.
2. Yin F, Sancheti H, Patil I, Cadenas E. Energy metabolism and inflammation in brain aging and Alzheimer’s disease, Free Rad Biol Med. 2016; 100, 108-122.
3. Arteaga de Castro C S, Boer V O, Andreychenko A, et al. Improved efficiency on editing MRS of lactate and γ-aminobutyric acid by inclusion of frequency offset corrected inversion pulses at high fields. NMR Biomed. 2013; 26, 1213–1219.
4. Boer V O, van Lier A L H M W, Hoogduin J M, et al. 7-T 1H MRS with adiabatic refocusing at short TE using radiofrequency focusing with a dual-channel volume transmit coil. NMR Biomed. 2011; 24, 1038–1046.
5. Tkac I, Andersen P, Adriany G, et al. In vivo 1H NMR spectroscopy of the human brain at 7T. Magn. Reson. Med. 2001; 46, 199–251.
6. Dev S I, Moore R C, Soontornniyomkij B, et al. Peripheral inflammation related to lower fMRI activation during a working memory task and resting functional connectivity among older adults: a preliminary study. Int. J. Geriatr. Psychiatry. 2017; 32, 341–349.
7. Dufouil C, Satizabal C L, Tzourio C, et al. Circulating IL-6 and CRP are associated with MRI findings in the elderly: The 3C-Dijon Study. Neurology. 2012; 78, 720–727.
8. Warren K N, Beason-Held L, Carlson O, et al. Elevated Markers of Inflammation Are Associated with Longitudinal Changes in Brain Function in Older Adults. Journals Gerontol. - Ser. A Biol. Sci. Med. Sci. 2018; 73, 770–778.
9. Wersching H, Duning T, Lohmann H, et al. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology. 2010;74, 1022–1029.
Figures