1851
Human cerebral blood flow-metabolic uncoupling during acute hypoxia: A 1H Magnetic Resonance Spectroscopy & Arterial Spin Labelling study1The Bangor Imaging Centre, School of Psychology, Bangor University, Bangor, United Kingdom, 2Extremes Research Group, School of Sport Health and Exercise Science, Bangor University, Bangor, United Kingdom
Synopsis
Acute exposure to a moderate hypoxic environment leads to regional specific reduction in cerebral blood flow (rCBF) to the posterior cingulate cortex. Despite this, there is no concomitant reduction in the main excitatory neurotransmitter glutamate, as would be expected if the reductions in rCBF were coupled to neural activity. Our findings obtained using a combined 1H Magnetic Resonance Spectroscopy and Arterial Spin Labelling paradigm indicate that hypoxia disrupts neurovascular coupling, as it is presently understood, in a regionally specific manner.
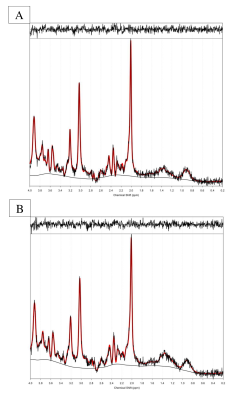
Methods: The present study exposed 11 participants to a moderate hypoxic environment (Fraction of inspired oxygen [FIO2] = 0.12) for 3.5 hours and a procedurally matched normoxia condition (FIO2 =.209). After 2 hours resting in an environmental chamber, participants were placed into a 3 T Philips Achieva MRI scanner whilst remaining in the hypoxic or normoxic condition, respectively. Whole brain resting state microvascular perfusion was quantified by pulsed Arterial Spin Labelling (22 slices, 2 x 2 x 5 mm in plane resolution, 1600 ms delay, TR 3 s, TE 15 ms, ), the regional resting state neurochemical environment was measured by PRESS 1H Magnetic Resonance Spectroscopy (MRS), (TE = 40 ms, TR = 2 s, voxel size 20 x 20 x 20 mm3) within the PCC. Perfusion maps were calculated using the BASIL (5) software package and registered to MNI space for group comparison using FLIRT (6). MRS spectra were analysed using TARQUIN (7) 4.3.10, referencing to an unsuppressed water peak for metabolite quantification. All metabolite concentrations were corrected using partial volume correction for grey matter, white matter and CSF before comparison.
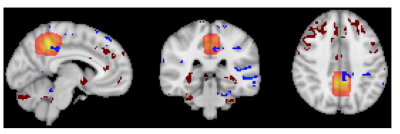
Results: Paired samples t-tests (cluster mass FWE correction at P<0.05) of the ASL data confirmed a reduction in perfusion during hypoxia compared to normoxia within the PCC and right posterior temporal cortex. In contrast MRS within the PCC revealed no significant change between normoxia M=15.76, SD=2.62 mM and hypoxia M=15.81, SD=3.91 mM in the excitatory neurotransmitter glutamate (p=0.9) nor any other major metabolite including creatine (p=0.7) , total n-acetyl aspartate (p=0.6) myo-inositol (p=0.7) and glutamine (p=0.3). However, a significant increase in Lactate was measured between normoxia M=0.57 SD=0.18 and hypoxia M=1.1, SD= 0.42, (p=0.01).
Conclusions: The present study supports our previous findings that indicate hypoxia induces a regional reduction in CBF within the PCC. Notably the PCC did not display a concomitant decrease in glutamate levels, however the measured increase in lactate would suggest an upregulation of non-oxidative metabolism. This indicates sustained neural activity and metabolic demand in presence of insufficient oxygen supply. Our data findings indicate this may be resulting from a hypoxia induced reduction in rCBF to the PCC without a concomitant reduction in neural activity. This challenges our present understanding of neurovascular coupling, whereby CBF is matched to demand, which is required to sustain healthy neural functioning. The findings suggest that hypoxia disrupts neurovascular coupling in a regionally specific manner, this may provide insight to specific cognitive deficits experienced at altitude.
Acknowledgements
Technical support and colleagues in the School of Psychology, Bangor University and the School of Sport Health and Exercise Sciences, Bangor University.References
1) Lawley, J. S., Macdonald, J. H., Oliver, S. J., & Mullins, P. G. (2017). Unexpected reductions in regional cerebral perfusion during prolonged hypoxia. The Journal of physiology, 595(3), 935-947.
2) Vestergaard, M. B., Lindberg, U., Aachmann-Andersen, N. J., Lisbjerg, K., Christensen, S. J., Law, I., ... & Larsson, H. B. (2016). Acute hypoxia increases the cerebral metabolic rate–a magnetic resonance imaging study. Journal of Cerebral Blood Flow & Metabolism, 36(6), 1046-1058.
3) Ip, I. Betina, Adam Berrington, Aaron T. Hess, Andrew J. Parker, Uzay E. Emir, and Holly Bridge. "Combined fMRI-MRS acquires simultaneous glutamate and BOLD-fMRI signals in the human brain." Neuroimage 155 (2017): 113-119.
4) Martínez-Maestro, M., Labadie, C., & Möller, H. E. (2018). Dynamic metabolic changes in human visual cortex in regions with positive and negative blood oxygenation level-dependent response. Journal of Cerebral Blood Flow & Metabolism.
5) Chappell, M. A., Groves, A. R., Whitcher, B., & Woolrich, M. W. (2008). Variational Bayesian inference for a nonlinear forward model. IEEE Transactions on Signal Processing, 57(1), 223-236.
6) Jenkinson, M., Bannister, P., Brady, M., & Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage, 17(2), 825-841.
7) Wilson, M., Reynolds, G., Kauppinen, R. A., Arvanitis, T. N., & Peet, A. C. (2011). A constrained least‐squares approach to the automated quantitation of in vivo 1H magnetic resonance spectroscopy data. Magnetic resonance in medicine, 65(1), 1-12.
Figures