1848
High temporal resolution glymphatic influx/efflux analysis with 3D-FISP sequence1Division of Glial Disease and Therapeutics, University of Copenhagen, Center for Translational Neuromedicine, Copenhagen, Denmark, 2Division of Glial Disease and Therapeutics, University of Rochester Medical Center, Center for Translational Neuromedicine, Rochester, NY, United States, 3Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark, Panum NMR Core Facility, Copenhagen, Denmark
Synopsis
The glymphatic system is a brain waste clearance pathway based on cerebrospinal fluid (CSF) and interstitial fluid exchange. As such, this pathway plays important roles in both acute and neurodegenerative conditions. With 3D-FISP-based fast imaging, we investigated how anesthetic regiments, i.e., ketamine-xylazine (K/X) versus isoflurane, influences the movement of tracer through the brain. This was achieved by conducting acute cisterna magna cannulations, tracer scanning using 3D-FISP, and calculating dynamic tracer distribution maps. Our analysis demonstrates that anesthesia alters the spatial and temporal distribution of CSF tracer, with K/X showing higher influx, and isoflurane shunting CSF out via deep cervical nodes.
Introduction
The glymphatic system is a functional waste clearance and metabolite transport route, which involves a dynamic exchange of cerebrospinal fluid (CSF) with interstitial fluid (ISF). Glymphatic clearance of metabolic waste products is central to normal brain physiology, while glymphatic dysfunction has been implicated in neurodegenerative diseases and accumulation of amyloid-β1. Dynamic contrast enhanced magnetic resonance imaging (DCE-MRI), in combination with the delivery of a small molecular weight paramagnetic contrast agent into the CSF, can be used to capture temporal and spatial characteristics of solute transport via the glymphatic pathway2. With DCE-MRI, the transport of paramagnetic contrast was tracked in the CSF and brain parenchyma over time through a time series of post-contrast enhanced images. The time series of T1 weighted (T1W) 2D/3D fast low angle shot (FLASH) sequence acquired during and after paramagnetic contrast delivery into the CSF space were previously used to assess the glymphatic function, however the temporal resolution of these sequences (4-5 minutes) is much slower than fluorescent optical imaging data. That is the one of the major drawbacks of DCE-MRI and important glymphatic kinetic parameters are missed due to the low temporal MRI resolution. Thus, a faster DCE-MRI approach is needed to characterize detail transport via the brain’s glymphatic system.Purpose
This study introduces a fast MR imaging technique to assess the glymphatic pathway based on DCE-MRI. With FISP-based rapid 3D DCE imaging approach, we investigated how the anesthetic regiment influences the movement of CSF tracer from the subarachnoid space into the perivascular spaces of the brain and ultimately into the parenchyma.Methods
This investigation was divided into two experimental conditions: isoflurane and ketamine-xylazine (K/X) anesthesia. In both experimental groups, the mice received an acute cisterna magna (CM) cannulation under the effect of their respective anesthetic. MRI was performed using a 9.4 Tesla preclinical scanner (BioSpec 94/30 USR, Bruker BioSpin, Ettlingen, Germany) equipped with a cryogenically-cooled quadrature-resonator (CryoProbe, Bruker BioSpin, Ettlingen, Germany). Mice were placed in the MR-compatible stereotactic holder in a prone position with ear bars to minimize head movement during scanning. Body temperature was maintained at 37 °C and monitored along with the breathing rate by a remote monitoring system (SA Instruments, NY, USA). T2-weighted rapid acquisition and relaxation enhancement scans (2D RARE: TR/TE: 9000/20ms, Matrix 192 × 128, FOV 19.2 mm × 12.8 mm, NEX 4, 76 sagittal slices, slice thickness = 0.2 mm) TOF-MRA (2D Flow-compensated FLASH: TR/TE: 15/3ms, Matrix 192 × 128, FOV 19.2 mm × 12.8 mm, NEX 4, 150 sagittal slices, slice thickness = 0.1 mm) and CSF cisternography with 3D-TrueFISP sequence (TR/TE 4.2/2.1 ms, Scan TR 848 ms, FA=50°, Matrix 256 × 192 × 192, FOV 19.2 × 14.4 × 14.4 mm, NEX 4) were performed. The contrast agent, gadobutrol (Gadovist, Bayer Pharma AG, Leverkusen, Germany) was infused into CM (12.5 mM gadobutrol at a constant rate of 1.5 µL/min for 8 min) followed by continuous MRI every 30 seconds for 60 min using the post excitation refocussed 3D Fast Imaging with Steady-state Free Precession (3D-FISP) sequence (TR/TE 3.26/1.63 ms, FA=15°, Single-shot, ScanTR=325.75ms, Matrix 192 × 128 × 128, FOV 19.2 × 12.8 × 12.8 mm, Nex 1). After the pre-processing, dynamic tracer distribution maps were calculated and brain uptake and clearance were measured in each brain segmentation.Results
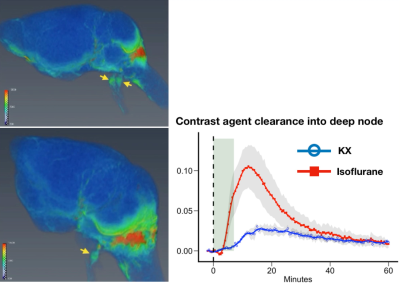
FISP-based DCE-MRI successfully demonstrated contrast agent distribution in the brain and that anesthesia alters the spatial and temporal distribution of CSF tracer with high temporal information. We found that glymphatic CSF tracer influx was higher under K/X compared to isoflurane (Figure1, 2). Tracer clearance via deep node can be seen under isoflurane anesthesia, but not under K/X (Figure3).Discussion
FISP is a gradient-echo technique with partially refocused transverse magnetization and have been developed for fast imaging especially for clinical use3. Postexcitation refocused steady-state amplitude delivers a T1-contrast, similar to the frequently used FLASH sequence4. In addition, 3D-FISP has potential benefits because of substantially higher signal-to-noise (SNR) and contrast-to-noise (CNR) compared to the 2D/3D-FLASH sequence. The utilization of 3D-FISP in combination with CSF contrast agent administration demonstrated that the tracer distributes not only in the brain but also in the clearance pathway around the skull base, including the nasal cavities and cranial nerve sheath. Following injection, tracer was shown to enter the parenchyma to a higher extent in the KX condition than under isoflurane. Interestingly, glymphatic flow under isoflurane was characterized by a distinct efflux pathway in which contrast agent was detected in the basal foramen and in the deep cervical lymph nodes. 3D-FISP is a particularly useful sequence to monitor the brain-wide distribution of CSF contrast agent with high spatiotemporal resolution.Conclusion
Imaging strategies for high-resolution 4D contrast mapping at 9.4T were developed, and whole-brain dynamic tracer distribution maps were derived from studying solute transport via the glymphatic system. This technique is sufficient to detect the glymphatic clearance pathway. FISP-based DCE-MRI offers a significant improvement over existing imaging approaches used to visualize the glymphatic system.Acknowledgements
No acknowledgement found.References
1) Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4(147):147ra111.
2) Iliff JJ. et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123:1299-1309.
3) Oppelt A, Graurann R, Barfuss H, et al. FISP: a new fast imaging sequence. Electromedica. 1986;54:15–18.
4) Chavhan GB, Babyn PS, Jankharia BG, et al. Steady-state MR imaging sequences: physics, classification, and clinical applications. Radiographics 2008;28(4):1147–1160.
Figures