1847
A Container and Detailed Preparation Protocol for Ex Vivo Whole Human Brain MRI1Biomedical Engineering and Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 3Graduate School of Biomedical Sciences, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 4Department of Pathology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 5Department of Rehabilitation Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 6Department of Neuroscience, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Synopsis
Whole-brain ex vivo MRI has proven extremely valuable in neuroscience research, but detailed descriptions of the specific preparation and packaging steps to minimize motion, susceptibility artifacts, and background signal are often missing from reports of experimental findings. We present the design of a reproducible container and a detailed protocol that yield whole-brain images uncorrupted by susceptibility artifacts. The most important steps to eliminate susceptibility artifacts due to residual air bubbles are removal of the leptomeninges, application of vacuum to dislodge bubbles, immersion in Fluorinert, and rolling of the sealed container, although removal of leptomeninges may be undesirable in some cases.
Introduction
Whole-brain or whole-hemisphere ex vivo MRI has yielded incredibly detailed information on neuroanatomy and neurological disorders1–5. However, detailed descriptions of the specific preparation and packaging steps to minimize motion, susceptibility artifacts, and background signal are, with few exceptions6–10, often omitted in reports focused on experimental findings. We therefore present the design of an easily reproducible container and a detailed protocol that yield whole-brain images almost completely free of susceptibility and motion artifacts.Methods
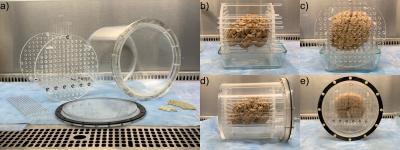
Container: Parts for an acrylic imaging container were cut from cylinder, sheet, and rod stock and assembled using acrylic welding solvent. The outer vessel has 8in outer diameter, 7.5in inner diameter, 8.625in outside length, and 8.25in inside length. The brain is stabilized by movable acrylic rods 7.875in long and 0.25in in diameter, anchored by two perforated end plates 0.4375in thick with 0.25in diameter holes at 0.5in on-center spacing. Three acrylic rods are permanently attached to one endplate, so that the endplate at the closed end of the vessel can be remotely manipulated without placing pressure on the brain tissue. The lid is constructed of three 0.156in thick acrylic plates, and is attached to a 0.5x0.5in flange on the vessel using twelve glass-filled nylon nuts and 1in long machine screws with 10-32 threads and 0.156in diameter. A 0.5in wide, 0.094in thick neoprene gasket provides a liquid-tight seal.Tissue preparation: Human brains were extracted and immersed in formalin for >1 month. Seven brains were scanned for the purpose of iteratively developing this protocol: five brains with intact leptomeninges; one hemi-brain with leptomeninges removed from the frontal lobe, slit over sulci in the temporal lobe, and intact in the parietal and occipital lobes; and one brain with leptomeninges removed. Each brain was agitated under a vacuum10 of -1 bar for 15min while immersed in formalin. A platform of rods was constructed between the two endplates, the brain was placed onto this platform, the endplates were slid against the brain, and additional rods were inserted to form a cage around the brain (Figure 1b,c). The caged brain was slid into the vessel, which was then filled with Fluorinert11,12 (FC-770, TMC Industries). Residual formalin, which is less dense than and immiscible with Fluorinert, was removed using a syringe, then the container was sealed and rolled through a full revolution >10 times to dislodge any remaining air and formalin.
Imaging: Imaging was performed on a 3T human scanner (Skyra, Siemens) using a 20-channel head/neck coil. The protocol included FLAIR (0.63mm isotropic, TR/TI/TE=4800/1650/291ms, 2.3hr), susceptibility-weighted imaging (0.63mm isotropic, TR/TE1/TE2=29.00/20.00/24.88ms, 55min), and five multi-echo GRE (ME-GRE) acquisitions (0.39mm isotropic, TR/TE1/TE2/TE3/TE4=28.0/2.95/8.80/14.60/20.40ms, FA=30°/25°/20°/15°/10°, 2hr each). ME-GRE images were combined by root-sum-of-squares, and T1, T2*, and apparent proton density (S0) maps were fitted.
Results
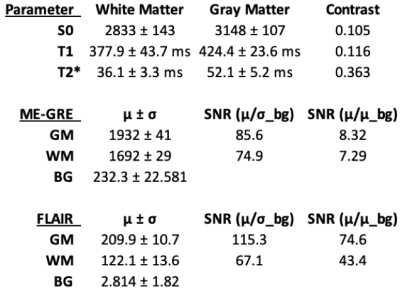
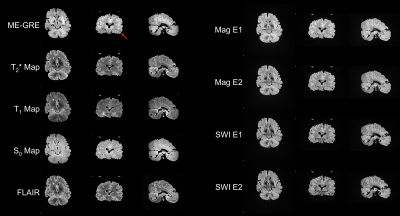
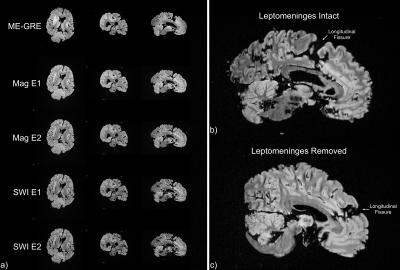
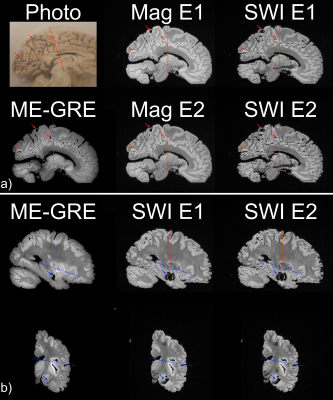
Relaxometry and SNR measurements are tabulated (Figure 2) in the best-prepared brain (Figure 3). When leptomeninges are removed, susceptibility artifacts arising from residual air bubbles in sulci are nearly completely eliminated; only a single bubble was visible (Figure 3). If the entire protocol is followed, except for removal of the leptomeninges, susceptibility artifacts overwhelm the images (Figure 4). In a hemi-brain in which portions of the leptomeninges were removed, slit, or left intact, more air bubbles remain in the areas covered by intact or slit leptomeninges than areas where the leptomeninges were removed (Figure 5).Discussion
The T1 and T2* values for formalin-fixed brain tissues are consistent with reported values13–15, providing additional confirmation that Fluorinert does not alter brain tissue’s relaxation properties. Fluorinert was chosen as the susceptibility-matching solution rather than Fomblin due to its lower viscosity, which allows it to fill the sulci and ventricles, but its low boiling point precludes its exposure to vacuum. Fluorinert also conducts heat more effectively than Fomblin, which mitigates tissue warming during long scans with high RF duty cycles.The echo-combined ME-GRE image provides the best resolution and yields parametric maps from the same data, but the acquisition time for this sequence is long. FLAIR provides excellent contrast and high resolution in a 2.3hr acquisition. The 20-channel head/neck coil and the longer RF wavelength at 3T provide excellent image uniformity.
Successful elimination of air is challenging and requires removal of the leptomeninges, which is a tedious process and is inevitably incomplete for deep fissures and the cerebellum. This step is also undesirable when preservation of the leptomeningeal-parenchymal interface is of histological interest. Slitting the leptomeninges over sulci was found to be no more effective in allowing air to escape than leaving the leptomeninges intact.
Opportunities remain to improve this container and protocol. There is little margin of error when tightening the screws to seal the container. If too loose, the container leaks; if overtightened, the threads strip. Larger fasteners or hardware made from a stronger material should be used in future designs. Also, the container does not fit into a standard 32ch 7T head coil. A smaller container with a hemispherical end would enable imaging at 7T, but these changes would significantly increase the complexity of manufacturing and assembling the container.
Conclusion
We have presented a simple and reproducible container design and preparation protocol that enables ex vivo human brain imaging at 3T with minimal susceptibility artifacts.Acknowledgements
This study was supported by NIH/NINDS U01-NS086625 (KDO).References
1. De Barros, A., Arribarat, G., Combis, J., Chaynes, P. & Péran, P. Matching ex vivo MRI With Iron Histology: Pearls and Pitfalls. Front. Neuroanat. 13, 68 (2019).
2. Yu, L. et al. Association Between Brain Gene Expression, DNA Methylation, and Alteration of Ex Vivo Magnetic Resonance Imaging Transverse Relaxation in Late-Life Cognitive Decline. JAMA Neurol 74, 1473 (2017).
3. Iglesias, J. E. et al. A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology. NeuroImage 183, 314–326 (2018).
4. Harper, L. et al. MRI visual rating scales in the diagnosis of dementia: evaluation in 184 post-mortem confirmed cases. Brain 139, 1211–1225 (2016).
5. Edlow, B. L. et al. Multimodal Characterization of the Late Effects of Traumatic Brain Injury: A Methodological Overview of the Late Effects of Traumatic Brain Injury Project. Journal of Neurotrauma 35, 1604–1619 (2018).
6. Shatil, A. S., Matsuda, K. M. & Figley, C. R. A Method for Whole Brain Ex Vivo Magnetic Resonance Imaging with Minimal Susceptibility Artifacts. Front. Neurol. 7, (2016).
7. Edlow, B. L. et al. 7 Tesla MRI of the ex vivo human brain at 100 micron resolution. http://biorxiv.org/lookup/doi/10.1101/649822 (2019) doi:10.1101/649822.
8. Augustinack, J. C. et al. MRI parcellation of ex vivo medial temporal lobe. NeuroImage 93, 252–259 (2014).
9. Miller, K. L. et al. Diffusion imaging of whole, post-mortem human brains on a clinical MRI scanner. NeuroImage 57, 167–181 (2011).
10. D’Arceuil, H. E., Westmoreland, S. & de Crespigny, A. J. An approach to high resolution diffusion tensor imaging in fixed primate brain. NeuroImage 35, 553–565 (2007).
11. Iglesias, J. E. et al. Effect of Fluorinert on the Histological Properties of Formalin-Fixed Human Brain Tissue. Journal of Neuropathology & Experimental Neurology 77, 1085–1090 (2018).
12. Pallebage-Gamarallage, M. et al. Dissecting the pathobiology of altered MRI signal in amyotrophic lateral sclerosis: A post mortem whole brain sampling strategy for the integration of ultra-high-field MRI and quantitative neuropathology. BMC Neurosci 19, 11 (2018).
13. Dawe, R. J., Bennett, D. A., Schneider, J. A., Vasireddi, S. K. & Arfanakis, K. Postmortem MRI of human brain hemispheres: T2 relaxation times during formaldehyde fixation. Magn. Reson. Med. 61, 810–818 (2009).
14. Raman, M. R., Shu, Y., Lesnick, T. G., Jack, C. R. & Kantarci, K. Regional T1 relaxation time constants in Ex vivo human brain: Longitudinal effects of formalin exposure. Magn. Reson. Med. 77, 774–778 (2017).
15. Shatil, A. S., Uddin, M. N., Matsuda, K. M. & Figley, C. R. Quantitative Ex Vivo MRI Changes due to Progressive Formalin Fixation in Whole Human Brain Specimens: Longitudinal Characterization of Diffusion, Relaxometry, and Myelin Water Fraction Measurements at 3T. Front Med (Lausanne) 5, 31 (2018).
Figures