1844
Clinical Application of Six-fold Accelerated Submillimeter Whole Brain 3D T2-weighted Imaging with Deep Learning Reconstruction1Department of Diagnostic Imaging and Nuclear Medicine, Kyoto University Graduate School of Medicine, Kyoto, Japan, 2MRI Systems Division, Canon Medical Systems Corporation, Kyoto, Japan, 3Human Brain Research Center, Kyoto University Graduate School of Medicine, Kyoto, Japan, 4Department of Advanced Medical Imaging Research, Kyoto University Graduate School of Medicine, Kyoto, Japan
Synopsis
We have demonstrated six-fold accelerated submillimeter whole brain 3D T2-weighted imaging with deep learning reconstruction (DLR) showed better coefficient of variation and signal ratio compared with that without DLR. Scan time of around 2.5 min is clinically feasible and the delineation of fine structure is preserved after DLR processing.
3D T2-weighted image with better image quality derived from DLR will help clinicians and radiologists evaluate CSF space abnormalities, and its short scan time will be feasible for routine clinical examinations.
Introduction
MR cisternography provides clinically useful information of cranial nerves and cerebrospinal fluid (CSF) space (1). Despite its usefulness, the use of MR cisternography is limited in cases for which some neurovascular compression syndrome and local cisternal abnormalities are clinically suspected. In particular, whole brain high resolution MR cisternography is usually difficult partly because the limitation of scan time.Two-dimensional parallel imaging is available for 3D MR imaging, and relatively high acceleration can be applied or image sequence, however, resultant imaging noise will be concerned because uniformly undersampled k-space data will lead to low-frequency artifact (2). Recently developed deep learning reconstruction (DLR) is expected to reduce noise with keeping imaging quality (3). Before clinical application DLR to whole brain MR cisternography, noise reduction, contrast ratio, and the visualization of cranial nerves should be evaluated.
In this study, we applied six-fold accelerated submillimeter 3D T2-weighted imaging with DLR and imaging quality and sharpness of cranial nerves were examined.
Methods
- Subjects
Local institutional committee approved this study. 19 patients were enrolled in this prospective observation study. Written informed consent was obtained. All patients underwent 3D T2-weighted imaging (Fast Advanced Spin Echo, FASE, an equivalent sequence of half Fourier single-shot fast spin echo) at MR unit (Vantage Galan 3T / ZGO, Canon Medical Systems Corporation, Otawara, Japan) with 32-channel head coil. - MR
image sequence
3D FASE: TR/TE, 2000/297.5 ms; constant flip angle, 89°; slice thickness, 0.7 mm, bandwidth 326 Hz/Px; field of view, 200 × 200 mm; matrix 352 × 352; resolution, 0.56 × 0.56 mm; acceleration factor of PE and SE, 3 × 2; scan time, 2 min 36 sec. - Postimaging process
- Coefficient of variation and signal ratio ROIs were placed on following structures: lateral ventricle (V_lat), white matter, fourth ventricle (V_4th), and pons (Figure 1). Homogeneity of signal intensity of cisterns and brain parenchyma was evaluated by coefficient of variation (CV, standard deviation divided by average signal) of each structure. Signal ratio (SR) of lateral ventricle/white matter, fourth ventricle/pons were also evaluated.
- Sharpness of trigeminal nerves Visualization of cisternal segment of cranial nerves is important in evaluation of neurovascular compression syndrome. A line was placed over trigeminal nerves and sharpness of trigeminal nerves were calculated as follows: gaussian curve fitting was conducted for the profile curve of the line. Sharpness was determined by the slope of 80% and 20 % signal values of fitted curve.
Results
Representative cases were shown in Figure 2 and 3.Coefficient of variation and signal ratio
Coefficient of variation (CV) of ventricles (V_lat and V_4th) were better in 3D-T2_DLR than 3D-T2 (Figure 4). CV of white matter (WM) and pons were also better in 3D-T2_DLR than 3D-T2 (Figure 4). In 3D T2-weighted imaging with a constant flip angle, the signal of CSF and brain parenchyma usually show homogeneous intensity. Low CV in ventricles, white matter and pons suggests less standard deviation for average signal intensity.
Signal ratio of V_lat/WM and that of V_4th/pons were better in 3D-T2_DLR than 3D-T2 (Figure 5). Better signal ratio between ventricles and white matter/pons in 3D-T2_DLR suggest the contrast among structures remain stable or even become better after DLR.
Sharpness of trigeminal nerves
Sharpness of trigeminal nerves was shown in Figure 5. Sharpness was not different between 3D-T2 and 3D-T2_DLR, which suggests DLR did not hamper the identification of fine structures such as trigeminal nerves.
Discussion and Conclusion
We have demonstrated Six-fold Accelerated Submillimeter Whole Brain 3D T2-weighted Imaging with DLR showed better CV and signal ratio compared with that without DLR. Scan time of around 2.5 min is clinically feasible and the delineation of fine structure is preserved after DLR processing.Visualization of finer structures such as arachnoid membrane is beneficial in neurosurgery, however, there are trade-offs between image resolution, imaging noise, and scan time. Higher resolution 3D-T2 with DLR may help us solve such trade-off problems, and further studies are required.
3D T2-weighted image with better image quality derived from DLR will help clinicians and radiologists evaluate CSF space abnormalities, and its short scan time will be beneficial for routine clinical examinations. However, further studies are required to demonstrate the clinical usefulness of 3D T2-weighted image with DLR.
Acknowledgements
No acknowledgement found.References
- Nakamura T, Naganawa S, Koshikawa T, et al. High-spatial-resolution MR cisternography of the cerebellopontine angle in 90 seconds with a zero-fill interpolated fast recovery 3D fast asymmetric spin-echo sequence. AJNR Am J Neuroradiol. 2002 Sep;23(8):1407-12
- Morita K, Nakaura T, Maruyama N, et al. Magn Reson Med Sci. Hybrid of Compressed Sensing and Parallel Imaging Applied to Three-dimensional Isotropic T 2 -weighted Turbo Spin-echo MR Imaging of the Lumbar Spine.
- Kidoh M, Shinoda K, Kitajima M, et al. Deep Learning Based Noise Reduction for Brain MR Imaging: Tests on Phantoms and Healthy Volunteers. Magn Reson Med Sci. 2019 Sep 4. doi: 10.2463/mrms.mp.2019-0018.
Figures
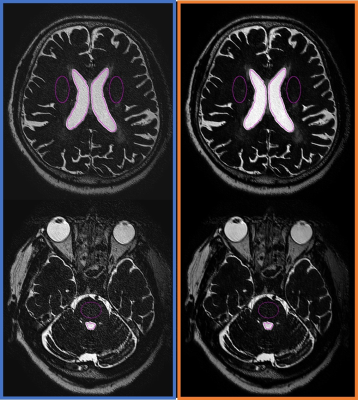
Figure 1.
ROIs were placed on following structures: lateral ventricle (V_lat), white matter, fourth ventricle (V_4th), and pons.
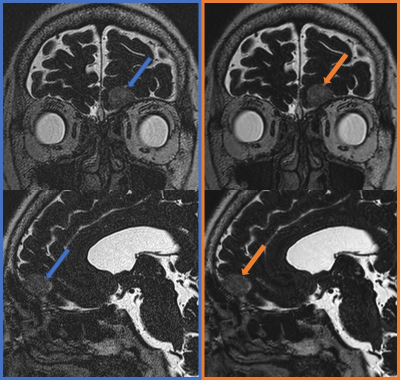
Figure 2.
A patient with left anterior skull base meningioma. 3D T2WI (left column, blue) and 3D T2WI with DLR (right column, orange). Multiplanar reconstructed coronal images (upper row) and sagittal images (lower row) showed the meningioma (arrows). We found less noise in 3D T2WI with DLR compared with 3D T2WI.
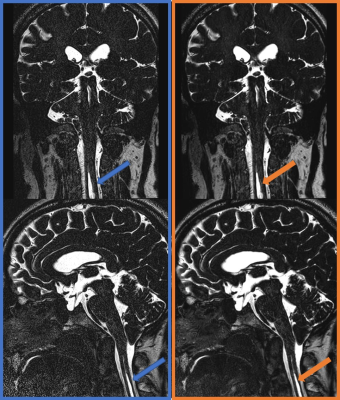
Figure 3.
A patient with Chiari malformation type 1. 3D T2WI (left column, blue) and 3D T2WI with DLR (right column, orange). Multiplanar reconstructed coronal images (upper row) and sagittal images (lower row) showed the syringomyelia in the cervical spinal cord (arrows). Fine structures are well visualized in both images, however, we found less noise in 3D T2WI with DLR compared with 3D T2WI (left, blue).
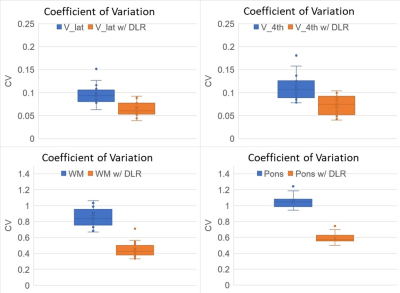
Figure 4.
Coefficient of variation (CV) of ventricles (V_lat and V_4th) were better in 3D-T2_DLR than 3D-T2 (Figure 4). CV of white matter (WM) and pons were also better in 3D-T2_DLR than 3D-T2 (Figure 4). In 3D T2-weighted imaging with a constant flip angle, the signal of CSF and brain parenchyma usually show homogeneous intensity.

Figure 5.
Signal ratio of V_lat/WM and that of V_4th/pons were better in 3D-T2_DLR than 3D-T2. Better signal ratio between ventricles and white matter/pons in 3D-T2_DLR suggest the contrast among structures remain stable or even become better after DLR.
Sharpness was not different between 3D-T2 and 3D-T2_DLR, which suggests DLR did not hamper the identification of fine structures such as trigeminal nerves.