1840
Multimodal Quantitative Magnetic Resonance Imaging in Adolescent Idiopathic Scoliosis
Bertwin Zhicheng Chen1, Reuben Chee Cheong Soh1,2, Septian Hartono2,3, Chu Ning Ann3, Ming-Ching Wen3, Weiling Lee1, Soo Lee Lim4, Julian Gan5, Yew Long Lo2,3, and Ling Ling Chan1,2
1Singapore General Hospital, Singapore, Singapore, 2Duke-NUS Medical School, Singapore, Singapore, 3National Neuroscience Institute, Singapore, Singapore, 4National Heart Centre Singapore, Singapore, Singapore, 5Siemens Healthineers, Singapore, Singapore
1Singapore General Hospital, Singapore, Singapore, 2Duke-NUS Medical School, Singapore, Singapore, 3National Neuroscience Institute, Singapore, Singapore, 4National Heart Centre Singapore, Singapore, Singapore, 5Siemens Healthineers, Singapore, Singapore
Synopsis
The aetiopathogenesis of adolescent idiopathic scoliosis (AIS) remains elusive, with studies suggesting a neurological basis. Using a case control, multimodal approach incorporating volumetry and diffusion tensor imaging, we characterised brain MRI changes in AIS. The pontine volume was significantly larger while left insula was smaller in patients than controls. Whole brain Tract-Based Spatial Statistics (TBSS) showed no difference between subject cohorts. Tractography of the corticoreticular pathway (CRP), which innervates the axial muscles, showed significant differences in mean and axial diffusivity between the left and right CRP only in the patient cohort, but no such asymmetry in the control cohort.
Introduction
Adolescent Idiopathic Scoliosis (AIS) is a progressive musculoskeletal disease characterised by a lateral spinal deformity of ≥ 10° affecting up to 4% of adolescents, with females predilection. AIS progression may lead to severe back pain requiring surgical intervention. Previous studies suggested a neurological aetiopathogenesis.1 The corticoreticular pathway, originating from the premotor cortex and terminating at the pontomedullary reticular formation, innervates axial muscles and may play a role in truncal muscular tone. In this study, we used a case control, multimodal quantitative approach incorporating brain volumetry and diffusion tensor tractography (DTT) to characterise brain and CRP changes on magnetic resonance imaging (MRI) in AIS.Methods
This study was approved by the local Centralised Institutional Review Board. Sixteen AIS patients and eighteen healthy controls (HC) were included in the study (Figure 1). A sex- and age-matched case-control study design was adopted. All participants underwent brain MRI using a 32-channel head coil on a 3T scanner including T1-weighted MPRAGE and Diffusion Tensor Imaging (DTI) sequences. The T1-weighted MRPAGE acquisition was performed with the following parameters: TR/TE/TI=2300/2.88/900 ms, matrix=256x256, resolution=1x1x1 mm3, GRAPPA factor=2, acquisition time=5:12 mins. DTI was performed with simultaneous multi-slice (SMS) imaging, in conjunction with readout-segmented (RESOLVE) echo-planar imaging (EPI)2 to reduce scan time and improve the quality of CRP tractography in the posterior fossa. The following parameters were used: TR/TE=3000/70 ms, RESOLVE factor=5, SMS factor=3, GRAPPA factor=2, diffusion directions=64 with b-value=0, 1000 s/mm2, matrix=128x128, resolution=2x2x2.5 mm3, acquisition time=16:10 mins.Brain volumetry was performed on MPRAGE images using the Siemens Morphobox prototype.3 Whole-brain tract-based spatial statistics (TBSS) was performed on DTI images to assess local changes in diffusion-based measures (fractional anisotropy-FA, mean, axial and radial diffusivity-MD, AD and RD, respectively) in voxel-wise analysis.4 DTT of the left and right CRP using deterministic tracking algorithm was also performed in DSI studio.5
CRP tractography was performed by two experienced MR technologists under the supervision of a neuroradiologist as per previously described protocol (Figure 2).6 A seed ROI was placed on the reticular formation of the medulla, with the first target ROI on the midbrain tegmentum and second target ROI on the pre-motor cortex. Student’s t-tests were carried out to compare DTI indices of the CRP between AIS patients and HC. Intra- and inter-rater reliability were assessed by intraclass correlation coefficient (ICC).
Results
There was no significant difference between AIS patients and HC with regards to age, gender composition, body mass index (BMI), handedness, and years of education (Figure 1).Pons volume was significantly bigger in AIS patients (1.42 ± 0.12%) compared to HC (1.31 ± 0.10%; p=0.006), whilst left insula volume was slightly smaller (AIS: 0.55 ± 0.04%, HC: 0.57 ± 0.02%; p=0.041). No significant differences in DTI measures were found between AIS patients and HC for all white matter tracts in TBSS.
Sample image of CRP vis-à-vis corticospinal tract (CST) was shown in Figure 3. ICC for DTT of the CRP was excellent (> 0.9) for both intra and inter-rater reliability. No significant difference was found for all diffusion indices in both left and right CRP between AIS patients and HC (Figure 4). However, there was a significant difference in mean (p=0.019) and axial diffusivity (p=0.013) between the left and right CRP in the patient cohort (Figure 5). Such CRP asymmetry was absent in the HC cohort (Figure 5).
Discussion
Our findings of relative pontine hypertrophy are consistent with findings from previous studies of a larger brainstem in AIS patients than HC.7 The primary site of pathology in AIS has been postulated to reside in the brainstem. Posture, proprioception, and equilibrium control functions are integrated by structures in and around the brainstem. Abnormality of the paramedian pontine reticular formation linking preocular motor nuclei and vestibular nuclei are suspect in AIS patients. Preclinical studies have also shown that creating lesions in the pons and periaqueductal grey matter could induce scoliosis.RESOLVE-DTI reduced geometric distortion from susceptibility artefacts and improved spatial resolution in the posterior fossa, affording better visualization of the small brainstem nuclei for accurate ROI placement in the medullary reticular formation in our study. Our FA and MD values of the CRP in HC are congruent with those reported in the literature.6,8 The CRP is responsible for innervation of both axial and proximal muscles required for gross motor skills such as postural control and locomotion.8 Diffusional asymmetry between left and right CRP in our AIS cohort are also congruent with findings of inter-side imbalance from intraoperative transcranial electrical stimulation studies in AIS patients showing primary, simultaneous asymmetric descending motor outputs and ascending sensory inputs, as opposed to that in adult degenerative scoliosis.1 In contrast, whole brain approach using TBSS analysis showed no differences between AIS patients and HC for all white matter tracts.
Conclusion
We found relative pontine hypertrophy and asymmetry of diffusion indices in the CRP in AIS patients, suggesting a primary brainstem origin to the source of inter-side axial neuromuscular imbalance in AIS. Larger scale neuroimaging studies could aid in further elucidating the complex aetiopathogenesis in AIS.Acknowledgements
We would like to thank National Neuroscience Institute, Singapore for their funding support.References
- Lo YL, Teo A, Tan YE, et al. Motor and somatosensory abnormalities are significant etiological factors for adolescent idiopathic scoliosis. Journal of Neurological Sciences. 2015; 359(1-2):117-123.
- Lim SL, Lee W, Chan LL, et al. The Many-Sidedness in using RESOLVE. MAGNETOM Flash ASEAN Edition. 2018; 01(7/2018):1-10.
- Schmitter D, Roche A, Maréchal B, et al. An evaluation of volume-based morphometry for prediction of mild cognitive impairment and Alzheimer’s disease. NeuroImage: Clinical. 2015; 7:7-17.
- Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006; 31:1487-1505.
- Yeh F-C, Verstynen TD, Wang Y, et al. Deterministic diffusion fiber tracking improved by quantitative anisotropy." PLoS ONE. 2013; 8(11):e80713.
- Yeo SS, Chang MC, Kwon YH, et al. Corticoreticular pathway in the human brain: Diffusion tensor tractography study. Neuroscience Letters. 2012; 508(1):9-12.
- Liu T, Chu WCW, Young, G, et al. MR Analysis of Regional Brain Volume in Adolescent Idiopathic Scoliosis: Neurological Manifestation of a Systemic Disease. Journal of Magnetic Resonance Imaging. 2008; 27(4):732-736.
- Yeo SS, Jang SH and Son SM. The different maturation of the corticospinal tract and corticoreticular pathway in normal brain development: diffusion tensor imaging study. Frontiers in Human Neuroscience. 2014; 8(573):1-6.
Figures
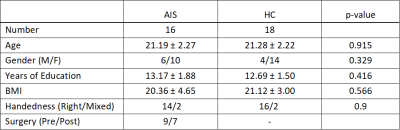
Figure 1. Study demographics.
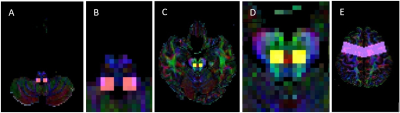
Figure
2. ROIs placement for CRP tract
generation. A seed ROI was placed on the
reticular formation of the medulla (A, magnified image: B). The first target
ROI was placed on the midbrain tegmentum (C, magnified image: D). The second
target ROI was placed on the pre-motor cortex (E).
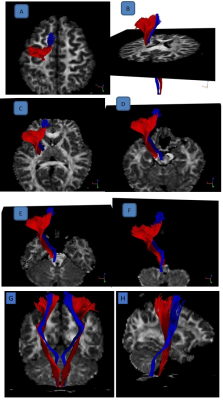
Figure 3. Blue: Corticoreticular pathway (CRP) and Red:
Corticospinal tract (CST). The entire pathway of CRP was validated with
reference to CST and illustrated through multiple axial slices. CRP
originates from the premotor cortex (A), and descends through the corona
radiata and the posterior limb of the internal capsule anterior to CST (B,C). It passes through the mesencephalic tegmentum in the medio-posterior
direction, traverses the pontine reticular formation (D,E), and
terminates at the pontomedullary reticular formation (F). Also shown: coronal (G) & sagittal (H) view.
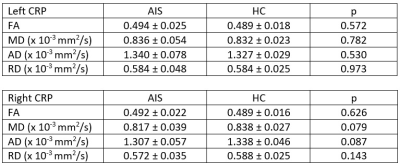
Figure 4. Comparison of DTI metrics
between AIS patients and healthy controls. Values shown are mean ± SD.
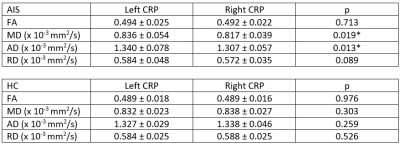
Figure 5. Comparison of DTI metrics between
left and right CRP in AIS patients and healthy controls. Values shown are
mean ± SD. *) p < 0.05.