1837
Quantitative mapping of brain tissue oxygenation around neural interfaces1Biomedical engineering, Arizona State University, Tempe, AZ, United States
Synopsis
There is a significant need to spatially map the pO2 levels around chronically implanted neural devices to assess the impact on surrounding tissue. Here we apply a quantitative MRI based oximetry approach to map pO2 around neural interfaces. Electrodes were coated with polydimethylsiloxane matrix and soaked in tetradecamethylhexasiloxane, which was characterized and used as a 1H MR pO2 reporter along with the previously developed PISTOL technique. Tissue pO2 in response to air and oxygen breathing was successfully measured from around implanted electrodes. Future studies will combine single-unit electrophysiology with quantitative, high resolution spatial maps of pO2 levels in vivo.
Introduction
It is known that long term signal reliability of chronically implanted neural devices gradually decreases over 3-6 months in rodent models and is significantly associated with the loss of neurons near the recording site. It is hypothesized that the diminishing levels of chronic neural activity near the electrode is in part due to changing dynamics of oxygen permeability around the implant site [1]. Since neural activity and function is dependent on oxygen for energy metabolism, there is a significant need to spatially map the dynamic changes in pO2 levels around implants over time. Current state-of-the art methods used to quantitate tissue oxygenation levels include, but not limited to, BOLD (blood-oxygen-level-dependent) signal in functional magnetic resonance imaging (fMRI), photoacoustic tomography, near infrared spectroscopy. These methods have significant tradeoffs in resolution or penetration depth which is critical in deep brain structure studies, furthermore, these techniques typically rely on indirect methods (i.e. presence/absence of hemoglobin levels) to measure tissue oxygenation. Recently, Kodibagkar et.al proposed a tissue oximetry technique, an 1H MRI based approach, known as proton imaging of siloxanes to map tissue oxygenation levels (PISTOL) [2]. Key advantages of PISTOL can be summarized as 1. Direct measure of oxygen level, 2. Quantitative mapping of pO2 at microscale, 3. Potential for adaptation for clinical use. The primary objective of this study is to quantitatively map the tissue oxygenation levels in the microenvironment surrounding implanted microprobes using PISTOL technique.Materials and methods
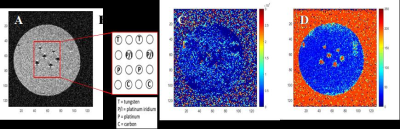
All animal studies were approved by Arizona State University’s Institute of Animal Care and Use Committee (IACUC). Tetradecamethylhexasiloxane (L6) with molecular weight of 459 g/mol was used as an MRI pO2 sensor. To assess the susceptibility artifacts, electrodes were made with 4 materials and coated with Polydimethylsiloxane (PDMS): tungsten, platinum-iridium, platinum and carbon fiber in epoxy. After electrode coating, they were soaked in L6 for 24h to reach saturation. All the electrodes were produced with diameter of 300 µm. The pO2 calibration curve for L6 was obtained by flowing different levels of oxygen (0, 5%, 10 %, 15% and 21% O2) in to a custom phantom containing L6. To assess signal sensitivity, an array of 4x4 PDMS pillars was soaked with L6 and embedded in agar. Finally, a 2x2 PDMS coated carbon electrode array was implanted in mouse brains (C57Bl6 wildtype, 30-40g). A large craniotomy (~3.5 - 4.0mm) was made on either side of the hemisphere centered approximately 3.5 mm posterior to bregma and 3.5 lateral to midline. The array was implanted up to 2.5 mm depth into the brain using a stereotaxic frame and sealed using gelfoam followed by bone cement. The imaging of the animals was 3.5 to 4 hours post implantation. MRI experiments were conducted on a preclinical 7T scanner, with a surface coil. After obtaining anatomical scouts, the position of the implanted electrode was identified in axial and coronal orientations using the chemical shift artifact from the proton resonance (0 ppm) of L6. Siloxane frequency selective spin echo and PISTOL sequences were used to confirm electrode position and map the T1 of the L6, respectively. The PISTOL sequence [2] consists of a combination of pulse-burst saturation recovery with frequency-selective excitation of the siloxane resonance that allows for the selective T1 mapping of the siloxane, which correlate with pO2 values [3]. Scanned images were subsequently analyzed using custom MATLAB codes, with conversion of T1 to pO2 based on the calibration experiment.Results
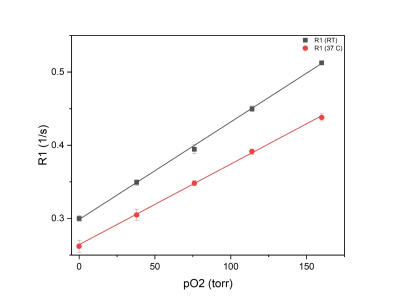
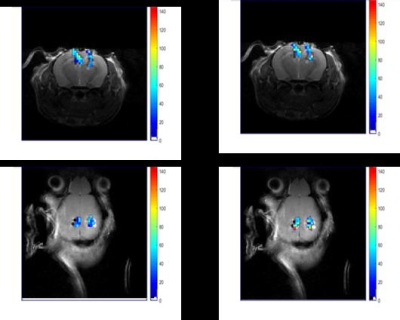
Figure1 shows the effect of material susceptibility on the MR images. Significant signal loss was seen around the implants for platinum and platinum-iridium electrodes while the tungsten electrodes showed a smaller effect and carbon showed no artifact. Based on this information, carbon-based electrodes were chosen for in vivo studies. Sufficient signal to noise ratio of the siloxane resonance was acquired from 200 µm X 200 µm X 500 µm voxels at 7T. Figure 3 demonstrates the calibration curve of R1 vs. pO2 for L6 at two temperature points of room temperature (RT) and 37˚C. The fit equations were R1= 0.29 s-1 + 0.0013 s-1.torr-1 * pO2 (RT) and R1= 0.26 s-1+ 0.0011 s-1.torr-1 *pO2 (37˚C). Next, in vivo oximetry was success demonstrated on mice (n=4) around the implanted electrodes. The pO2 levels of the animals’ brain tissue were measured under air and oxygen breathing. Figure 4 shows the changes in pO2 levels around the electrodes after switching the breathing gas from air to pure medical O2 after 10 min. While individual electrodes showed increases in pO2 on oxygen breathing, the group means over the cohort were not significantly different (p>0.05). Data are summarized in Table1.Conclusion
This study demonstrates that PISTOL MR imaging technique can be useful for measuring tissue oxygenation around microscale electrodes used as brain implants. L6 was characterized for the first time in this study as a promising pO2 reporter probe. This study shows L6-PDMS coated carbon electrodes along with quantitative tissue oximetry can potentially be used for functional brain imaging in conjunction with microelectrode recordings. The lack of significant changes in pO2 around neural implants in response to administration of 100% oxygen can be due to disruption of local vasculature or blood flow in the vicinity of the implants.Acknowledgements
These studies were supported by UF1NS107676 from the NIH Brain Initiative.References
1. Wellman SM, Kozai TDY. Understanding the Inflammatory Tissue Reaction to Brain Implants To Improve Neurochemical Sensing Performance. ACS Chemical Neuroscience. 2017;8(12):2578-2582.
2. Kodibagkar VD, Wang X, Pacheco-Torres J, Gulaka P, Mason RP. Proton imaging of siloxanes to map tissue oxygenation levels (PISTOL): a tool for quantitative tissue oximetry. 2008;21(8):899-907.
3. Kodibagkar VD, Wang X, Mason RP. Physical principles of quantitative nuclear magnetic resonance oximetry. Front Biosci. 2008;13:1371-84
Figures