1830
Development of simultaneous calcium photometry and fMRI of the mouse brain
Shabnam Khorasani Gerdekoohi1, Hsu-Lei Lee1, Helena Hung-Yin Huang1, Pankaj Sah1, and Kai-Hsiang Chuang1,2
1Queensland Brain Institute, University of Queensland, Brisbane, Australia, 2Centre for Advanced Imaging, University of Queensland, Brisbane, Australia
1Queensland Brain Institute, University of Queensland, Brisbane, Australia, 2Centre for Advanced Imaging, University of Queensland, Brisbane, Australia
Synopsis
Neural calcium activity of genetic reporter GCaMP6f was successfully recorded in 9.4T MRI.
Event-related BOLD activation can be measured using short stimuli.
Susceptibility artefacts from the optic fiber and dental cement affect fMRI quality.
Introduction
Optical recording of neural activity, such as calcium, provides versatile and important information for understanding the neural basis of functional MRI measures of neural activity and functional connectivity. Optic fiber photometry has been shown to be compatible with fMRI of the rat brain (1–4). However, its use in the mouse brain remains challenging due to image artefact and low signal in the mouse brain. A recent study uses high sensitivity photo detector with an optic fiber mounted over the skull to record large but slow GCaMP6s activity from the superficial layer of the somatosensory cortex while minimizing artifact on a 7T MRI (5). In this study, we adopted a camera-based photometry that allows multi-channel recording (6) with fiber implanted into the cortex and subcortical nuclei. To evaluate the feasibility to measure transient response, an event-related design was adopted using short stimulus together with a fast but weak calcium reporter, GCaMP6f, and ultrafast fMRI (7).Methods
The study was approved by the animal ethic committee of the University of Queensland. Young C57BL6/J mice were stereotaxically injected with AAV-hSyn-GCaMP6f-P2A-NLS-tdTomato into either the primary visual cortex (V1) or lateral genicular nucleus (LGd) to drive the expression of calcium reporters in the neurons. An optic fiber cannula (200um, NA=37) was then implanted and secured by dental cement. After 4-6 weeks of recovery, functional study was conducted under 0.1mg/kg/h medetomidine and 0.25-0.5% isoflurane anesthesia. Visual evoked responses to a brief visual stimulation (3s duration, 5Hz, 20ms pulse, 12s inter-trial-interval, 15 trials) were measured by a home-built photometry system and by a 9.4T MRI with 10mm single loop receive coil (Bruker). BOLD fMRI was acquired using GE-EPI (TR=1s, TE=15ms) and an ultrafast multiband EPI (TR=0.3s, TE=15ms) covering the whole brain (7). The calcium signal was recorded by a CMOS camera (ThorLab) with an excitation light (470nm, 10mW/mm2) alternated with a reference light (410nm) sampled at 20Hz frame rate (10Hz per wavelength). FMRI signal was analyzed by FSL and time-courses were extracted from regions-of-interest selected in V1 or LGd. The calcium signal was processed by regressing the reference signal to remove background fluorescence change. The averaged responses over the repeated trials were compared.Results
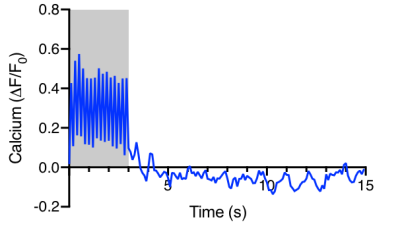
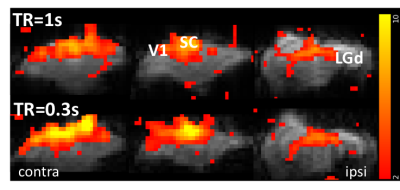
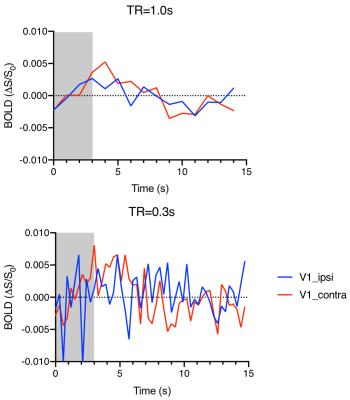
Fig.1 shows the averaged GCaMP6f signal in the V1. With the fast reporter, the response to each stimulus light pulses at 5Hz can be clearly differentiated. It also showed no adaption to the short stimuli. Strong BOLD activation can be detected in the visual pathway by both TR (Fig.2). Multiband EPI provided greater sensitivity but suffered more geometric distortion. The EPI signal around the optic fiber implant was highly attenuated due to larger field change over the small mouse brain. BOLD responses measured at 1 and 0.3 s TR showed 3-4 s delay time to peak. Although the signal of TR=1s appeared to be less noisier, the response detected at TR=0.3s delineated more dynamic changes.Discussion
Simultaneous calcium photometry and fMRI was successfully implemented in measuring event-related responses in the mouse visual pathway. The photometry system shows good sensitivity to the fast GCaMP6f signal. So far, most rodent fMRI studies have been conducted using block design to maximize the detectability. We demonstrated that both single and multi-band EPI protocols are suitable for event-related fMRI with multiband EPI depicted more transient change that would be useful for deriving the hemodynamic response function and the coupling function with the calcium activity. Further study is ongoing to reduce the susceptibility artefacts (signal dropout and distortion) from the optic fiber implant and dental cement by optimizing materials, pulse sequences, etc.Acknowledgements
No acknowledgement found.References
- Wang M, He Y, Sejnowski TJ, Yu X. Brain-state dependent astrocytic Ca2+ signals are coupled to both positive and negative BOLD-fMRI signals. Proc Natl Acad Sci U S A. 2018;115(7):E1647–E1656.
- Schulz K, Sydekum E, Krueppel R, et al. Simultaneous BOLD fMRI and fiber-optic calcium recording in rat neocortex. Nat Methods. 2012;9(6):597–602
- Schmid F, Wachsmuth L, Schwalm M, et al. Assessing sensory versus optogenetic network activation by combining (o)fMRI with optical Ca2+ recordings. J Cereb Blood Flow Metab. 2016;36(11):1885–1900
- Liang Z, Ma Y, Watson GDR, Zhang N. Simultaneous GCaMP6-based fiber photometry and fMRI in rats. J Neurosci Methods. Elsevier B.V.; 2017;289:31–38
- Schlegel F, Sych Y, Schroeter A, et al. Fiber-optic implant for simultaneous fluorescence-based calcium recordings and BOLD fMRI in mice. Nat Protoc. 2018;13(5):840–855
- Kim CK, Yang SJ, Pichamoorthy N, et al. Simultaneous fast measurement of circuit dynamics at multiple sites across the mammalian brain. Nat Methods. 2016;13(4):325–328
- Lee H-L, Li Z, Coulson EJ, Chuang K-H. Ultrafast fMRI of the rodent brain using simultaneous multi-slice EPI. Neuroimage. Elsevier Inc.; 2019;195:48–58