1823
Detection of microstructural abnormalities of gray matter in migraineurs without aura using Mean Apparent Propagator (MAP) MRI model1Radiology, union hospital, tongji medical college, Huazhong University of science and technology, Wuhan, China, 2Neurology, union hospital, tongji medical college, Huazhong University of science and technology, Wuhan, China, 3MR Scientific Marketing, Siemens Healthcare, Shanghai, China, 4Shanghai Key Laboratory of Magnetic Resonance,East China Normal University, Shanghai, China
Synopsis
The mean apparent propagator (MAP) MRI is an advanced diffusion model proposed recently for brain microstructure imaging. In this study, the MAP-MRI model was applied in diagnosis of migraineurs patient without aura. The results found that the parameter of MAP-MRI showed significant differences in in superior frontal gyrus, temporal lobe, cingulate gyrus, parahippocampal gyrus, hippocampus and left amygdata in MWoAs between patients and healthy controls. These findings further suggest involvement of the gray matter in pathology of migraine without aura.
INTRODUCTION
Recent studies showed that microstructural changes in gray brain regions are found in migraineurs without aura (MWoAs) using diffusion MRI 1 2. Meanwhile, mean Apparent Propagator (MAP)-MRI is an advanced diffusion model proposed recently, which represents a new comprehensive framework for modeling 3-D q-space signals and converting them into diffusion propagators to describe the molecular displacement 3. Some studies demonstrated that MAP-MRI could provide novel and quantitative parameters to reflect changes of neural tissue microstructures 4 5. Our aim is to explore the diagnostic performance of MAP-MRI in detecting microstructure abnormalities in gray matter of MWoAs.METHODS
This study was approved by the ethics committee of our hospital. Totally 25 MWoAs were recruited based on the International Classification of Headache Disorders 3rd edition criteria 6, and were scanned during an interictal period. 25 age- and gender-matched healthy controls (HCs) were enrolled. All subjects underwent Magnetization Prepared Rapid Gradient Echo (MP2RAGE) and diffusion spectrum imaging (DSI) sequences on a 3T MR scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany). The DSI parameters were as follows: TR/TE= 5200/111 ms, FOV = 220×220 mm2, slice thickness = 2 mm, voxel size = 2×2×2 mm3, GAPPA = 2, slice accelerated factor= 2, Δ/ δ= 55.2/32.2 ms, q-space Cartesian grid sampling scheme is used with two b=0 and 98 diffusion images with different diffusion gradient directions and bmax= 3000 s/mm2. The eddy current distortion was corrected by bneddy tool of DiffusionKit software7. The MAP-MRI parameters were calculated using software developed in-house with Python, called NeuDiLab, which is based on an open-resource tool DIPY (Diffusion Imaging in Python, Tttp://nipy.org/dipy). The MAP-MRI parameters included the return-to-the-origin probability (RTOP), return-to-the-plane probability (RTAP), return-to-the-axis probability (RTPP), Q-space inverse variance (QIV), mean squared displacement (MSD). The mean values of the parameters in different brain regions were calculated based on anatomical automatic labeling (AAL) template using an in-house developed BrainQuan tool8. Paired-sample t-test was used to compare the difference between the patients with migraine and controls. P < 0.05 was considered statistically significant.RESULTS
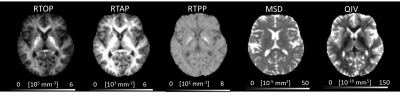
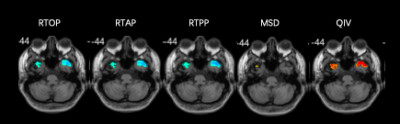
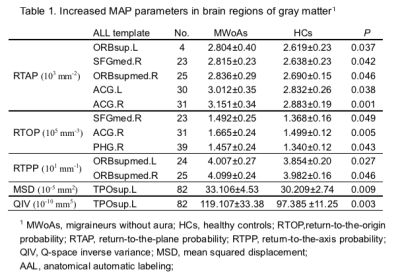
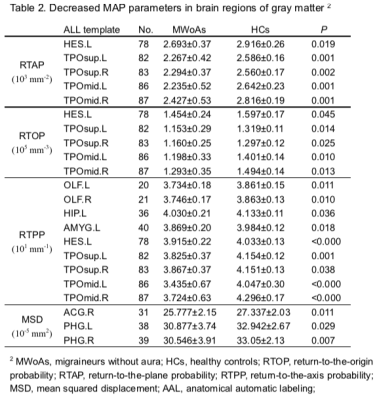
We included 25 MWoA and 25 age- and gender-matched controls (mean age±standard deviation, 27 years±10.1; range, 17-49 years; 23 female; 2 men). Figure 1 and figure 2 shows the parameter maps of one healthy subject and migraineurs without aura, respectively. The RTOP, RTAP and RTPP in the bilateral superior frontal gyrus were significantly higher in the MWoAs than HCs (P< .05). MWoAs exhibited decreased RTPP in the left amygdata, hippocampus and bilateral olfactory cortex (P< .05).We also found lower the RTOP, RTAP and RTPP in the bilateral temporal pole and left heschi gyrus in the MWoAs (P< .05). The RTOP,RTAP in the bilateral anterior cingulate and paracingulate gyri were significant higher in the MWoAs than HCs, while the MSD in the right these regions were lower than HCs (P< .05). The RTOP in the right para-hippocampal gyrus were significant higher in the MWoAs than HCs, while the MSD in the bilateral these regions were lower than HCs (P< .05). The detailed results are listed in Table 1 and Table 2.DISCUSSION
The parameters of MAP-MRI model showed significantly differences in superior frontal gyrus, temporal lobe, cingulate gyri and left amygdata, which are associated with pain pathway. The bilateral temporal pole showed more severe and widespread decreased in the family of zero-displacement probability measures (RTOP, RTAP, RTPP) compared with healthy controls, which calculated along and across the axis of the local reference frame determined by the diffusion tensor 3. Interestingly, bilateral superior frontal gyrus was observed increased in these parameters. Previous studies have found that the lower gray matter volume in the temporal lobe, frontal gyrus, the left amygdala and the hippocampus in migraine 9 10. Our results suggest that the process of the pain may is involved in different neural pathophysiological mechanisms in multiple brain regions. These structural differences could lead to modulation and conduction of harmful information, as well as integration dysfunction in migraine without aura. MAP-MR parameters may become more specific biomarkers of cellularity, cell size or presence of restricting barriers reflecting physically microstructural features.CONCLUSION
MAP-MRI can reveal microstructural changes in multiple brain regions between patients with migraine and healthy controls. These findings further suggest involvement of the gray matter in the pathology of migraine without aura.Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No.81701653)References
1. Qin Z, He XW, Zhang J, et al. Structural changes of cerebellum and brainstem in migraine without aura. J Headache Pain 2019;20(1):93. doi: 10.1186/s10194-019-1045-5 [published Online First: 2019/09/04]
2. Planchuelo-Gomez A, Garcia-Azorin D, Guerrero AL, et al. Structural connectivity alterations in chronic and episodic migraine: A diffusion magnetic resonance imaging connectomics study. Cephalalgia 2019:333102419885392. doi: 10.1177/0333102419885392 [published Online First: 2019/11/02]
3. Ozarslan E, Koay CG, Shepherd TM, et al. Mean apparent propagator (MAP) MRI: a novel diffusion imaging method for mapping tissue microstructure. Neuroimage 2013;78:16-32. doi: 10.1016/j.neuroimage.2013.04.016 [published Online First: 2013/04/17]
4. Avram AV, Sarlls JE, Barnett AS, et al. Clinical feasibility of using mean apparent propagator (MAP) MRI to characterize brain tissue microstructure. Neuroimage 2016;127:422-34. doi: 10.1016/j.neuroimage.2015.11.027 [published Online First: 2015/11/21]
5. Karmacharya S, Gagoski B, Ning L, et al. Advanced diffusion imaging for assessing normal white matter development in neonates and characterizing aberrant development in congenital heart disease. Neuroimage Clin 2018;19:360-73. doi: 10.1016/j.nicl.2018.04.032 [published Online First: 2018/07/18]
6. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018;38(1):1-211. doi: 10.1177/0333102417738202 [published Online First: 2018/01/26]
7. Xie S, Chen L, Zuo N, et al. DiffusionKit: A light one-stop solution for diffusion MRI data analysis. J Neurosci Methods 2016;273:107-19. doi: 10.1016/j.jneumeth.2016.08.011 [published Online First: 2016/10/25]
8. Xiang Feng MXL, Guang Yang, and Xu Yan,. BrainQuan: An integrated tool for automated and region-specific analysis of multi-parametric brain MRI data. Proc Intl Soc Mag Reson Med 2019:p4820.
9. Valfre W, Rainero I, Bergui M, et al. Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache 2008;48(1):109-17. doi: 10.1111/j.1526-4610.2007.00723.x [published Online First: 2008/01/11]
10. Coppola G, Petolicchio B, Di Renzo A, et al. Cerebral gray matter volume in patients with chronic migraine: correlations with clinical features. J Headache Pain 2017;18(1):115. doi: 10.1186/s10194-017-0825-z [published Online First: 2018/01/13]
Figures