1822
Multimodal quantitative magnetic resonance neurography in chronic inflammatory demyelinating polyneuropathy1Radiology, union hospital, tongji medical college, Huazhong University of science and technology, Wuhan, China, 2Neurology, Renming Hospital of Wuhan University,, Wuhan, China, 3MR Scientific Marketing, Siemens Healthcare, Shanghai, China
Synopsis
Conventional imaging is insufficient for diagnosis and management of patients with chronic inflammatory demyelinating polyneuropathy (CIDP). Brachial and lumbosacral plexus of 37 CIDP patients and 37 age and gender-matched controls were examined by using multi-sequences. Our study showed that fractional anisotropy (FA) had the most sensitivity, while contrast-enhanced ratio (CR) had the most specificity in single-parameter. The combination of FA and CR value had the best diagnostic efficiency. FA had a negative correlation with course duration, and CR positive correlation with CSF protein. SPACE combined with DTI and contrast enhancement sequences serve as a recommended composite protocol.
INTRODUCTION
Chronic inflammatory demyelinating polyneuropathy (CIDP) is an acquired immune-mediated and treatable demyelinating disorder of the peripheral nervous system 1,2. The diagnosis and management of CIDP can be difficult, and is mainly based on clinical features and nerve conduction studies 2-4. CIDP is most frequently observed in adult men, and has an annual incidence of 0.48 per 100,000 individuals in the population 5. The aims of this study were to evaluate the diagnostic performance and abnormalities of brachial and lumbosacral (LS) plexus via quantitatively multimodal MR imaging for CIDP, and to come up with optimal combined parameters. Concurrently, the potential correlations between clinical parameters with multi-parameters of MR were investigated.METHODS
A total of 37 typical patients with CIDP were recruited based on the European Federation of Neurological Societies / Peripheral Nerve Society diagnostic criteria. The Inflammatory Rasch-built Overall Disability Scale (I-RODS) questionnaire scores 6, disease duration and cerebrospinal fluid protein values were documented. Brachial and LS plexus of 37 CIDP patients and 37 age- and gender-matched healthy controls were examined on a 3T MR scanner (MAGNETOM Trio, Siemens Healthcare, Erlangen, Germany). The sequences included the volumetric interpolated breath-hold examination (VIBE), turbo inversion recovery magnitude (TIRM), sampling perfection with application-optimized contrasts using different flip angle evolution (SPACE) and diffusion tensor imaging (DTI). Nerve diameter, nerve-to-muscle T2 signal intensity ratio (nT2), contrast-enhanced ratio (CR), fractional anisotropy (FA) and apparent diffusion coefficient (ADC) were determined in brachial and LS plexus, and tractography was performed. Wilcoxon signed-rank test was used to assess the differences between the patients and controls. Receiver operating characteristic (ROC) curve analysis was used to determine the diagnostic efficiency. Spearman rank correlation test was performed to investigate for the correlations among parameters. Intraclass correlation coefficients (ICC) were calculated to the consistency of inter- and intra-observer. Two-tailed P < 0.05 was considered statistically significant.RESULTS
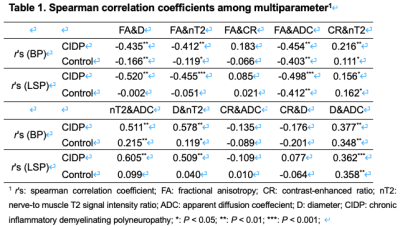
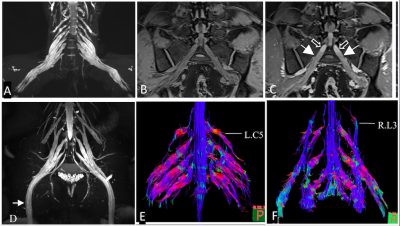
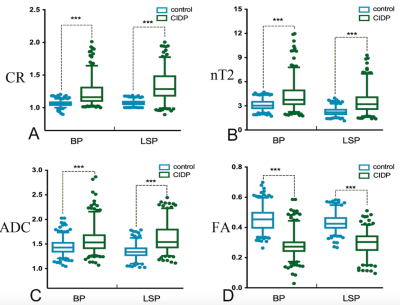
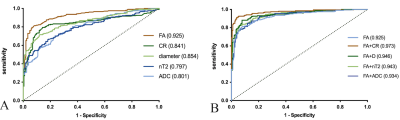
Hypertrophy involved the brachial (n=22/37) and LS (n=27/36) plexus in the CIDP patients, and no hypertrophy in controls. The representative hypertrophy, enhancement and diffusion tensor tractographies were illustrated as in the brachial and LS plexus of CIDP patients (Figure 1) The diameters of the C5-C8, L4-S1 nerve roots, sciatic and femoral nerves were significantly larger in CIDP patients than in healthy controls (all P< .01). As shown in Figure 2, CR, nT2 and ADC were significantly higher while the FA was lower in CIDP patients than in controls (P< .01). The AUC among each parameter are higher in the LS plexus than brachial plexus. The ROC curves of the single-parameter and combined parameters models in the LS plexus were showed in Figure 3. FA had the highest sensitivity (.809), accuracy (.73) and AUC (.925), while the highest specificity was .961 for CR in single-parameter in the LS plexus. The combination of FA and CR demonstrated the highest sensitivity (.922), specificity (.951), accuracy (.87) and AUC (.973) in the LS plexus. FA had a weak negative correlation with course duration (r= -.302, P=.04), and CR had a moderate positive correlation with CSF protein (r= .415, P=.01). No correlation was found between the I-RODs with MR parameters (P range, 0.33-0.91). CR only had a weak correlation with nT2. ADC and diameter had moderate positive correlation with nT2, and the diameter and nT2 had moderate negative correlation with FA in CIDP; while there was only a weak or no correlation between these parameters in controls. The correlation results are summarized in Table 1. There were good inter- and intra-observer consistencies (ICC range, 0.754-0.894) for each parameter assessed in the plexus.DISCUSSION
Although previous studies have exclusively reported DTI parameters alterations in the lower limb nerves in CIDP patients, 7,8 our study first assessed the diagnostic accuracy of DTI with tractography in the brachial and LS plexus, which complemented the results from the literature. FA value decrease is likely due to abnormal myelin development as a result of repeated loss, repair and axonal degeneration, which in turn leads to less molecule diffusion along long nerve axes. The tight junctions of blood-nerve barrier provide a barrier to the diffusion of various tracers with larger molecular weight 9. Early studies have indicated a decreased number of tight junction proteins claudin 5 and ZO-1 in the sural nerve biopsy specimens of CIDP patients 1,10. We reported the utility of contrast enhancement to quantify alteration of the BNB permeability in the plexus. Our results revealed that CR had the advantage of high specificity, and had a moderate positive correlation with CSF protein. CR is likely to be a potential non-invasive repeatable MR biomarker for detecting and monitoring disease activity.CONCLUSION
Multimodal MR imaging possesses a high diagnostic accuracy of CIDP in the LS plexus. SPACE combined with DTI and contrast enhancement serve as a recommended composite protocol for assessing demyelinating polyneuropathy.Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 81470076). We thank our statistician colleagues who helped the data analysis.
References
1. Vallat JM, Sommer C, Magy L. Chronic inflammatory demyelinating polyradiculoneuropathy: diagnostic and therapeutic challenges for a treatable condition. Lancet Neurol 2010;9(4):402-12. doi: 10.1016/S1474-4422(10)70041-7
2. Latov N. Diagnosis and treatment of chronic acquired demyelinating polyneuropathies. Nat Rev Neurol 2014;10(8):435-46. doi: 10.1038/nrneurol.2014.117
3. Rajabally YA, Stettner M, Kieseier BC, et al. CIDP and other inflammatory neuropathies in diabetes — diagnosis and management. Nature Reviews Neurology 2017;13(10):599-611. doi: 10.1038/nrneurol.2017.123
4. Querol L, Devaux J, Rojas-Garcia R, et al. Autoantibodies in chronic inflammatory neuropathies: diagnostic and therapeutic implications. Nat Rev Neurol 2017;13(9):533-47. doi: 10.1038/nrneurol.2017.84
5. Iijima M, Koike H, Hattori N, et al. Prevalence and incidence rates of chronic inflammatory demyelinating polyneuropathy in the Japanese population. J Neurol Neurosurg Psychiatry 2008;79(9):1040-3. doi: 10.1136/jnnp.2007.128132 [published Online First: 2008/01/29]
6. van Nes SI, Vanhoutte EK, van Doorn PA, et al. Rasch-built Overall Disability Scale (R-ODS) for immune-mediated peripheral neuropathies. Neurology 2011;76(4):337-45. doi: DOI 10.1212/WNL.0b013e318208824b
7. Lichtenstein T, Sprenger A, Weiss K, et al. MRI biomarkers of proximal nerve injury in CIDP. Ann Clin Transl Neurol 2018;5(1):19-28. doi: 10.1002/acn3.502
8. Kronlage M, Pitarokoili K, Schwarz D, et al. Diffusion Tensor Imaging in Chronic Inflammatory Demyelinating Polyneuropathy: Diagnostic Accuracy and Correlation With Electrophysiology. Invest Radiol 2017;52(11):701-07. doi: 10.1097/RLI.0000000000000394
9. Olsson Y, Reese TS. Permeability of vasa nervorum and perineurium in mouse sciatic nerve studied by fluorescence and electron microscopy. J Neuropathol Exp Neurol 1971;30(1):105-19.
10. Kanda T, Numata Y, Mizusawa H. Chronic inflammatory demyelinating polyneuropathy: decreased claudin-5 and relocated ZO-1. J Neurol Neurosurg Psychiatry 2004;75(5):765-9.
Figures