1818
Diffusion Imaging Correlates of Neurocognition in Healthy Adults: The Potentials of Sequences with Multiple Diffusion Encoding Schemes1Department of Diagnostic and Interventional Radiology, Hokkaido University Hospital, Sapporo, Japan, 2Global Station for Quantum Medical Science and Engineering, Hokkaido University, Sapporo, Japan, 3Department of Biomarker Imaging Science, Hokkaido University Graduate School of Biomedical Science and Engineering, Sapporo, Japan, 4Siemens Healthcare, Tokyo, Japan, 5Department of Rehabilitation, Hokkaido University Hospital, Sapporo, Japan
Synopsis
This prospective study was aimed to identify the diffusion imaging correlates of neurocognition. Newly developed diffusion-imaging sequences, i.e., oscillating gradient spin-echo (OGSE), pulsed gradient spin-echo (PGSE), and double diffusion encoding (DDE) sequences, were acquired in 30 healthy adults. The diffusion imaging indices were tested for correlation with the neuropsychological test scores. The microscopic fractional anisotropy (υFA) and fractional anisotropy (FA) of the right caudate nucleus, the υFA of the right temporal lobe, and the OGSE sequence-derived apparent diffusion coefficient (ADC) of the right sublobar white matter showed significant correlations with forward digit span test or trail-making test part-B.
Introduction
Diffusion imaging sequences have long been used to noninvasively assess the integrity of tissue microstructure in normal and pathological states1, 2. Of several indices, apparent diffusion coefficient (ADC) and fractional anisotropy (FA) are the two most frequently extracted diffusion imaging indices. These indices are traditionally extracted from diffusion-weighted (DWI) and diffusion tensor imaging (DTI) sequences, but the sensitivity of these sequences to detect subtle tissue microstructural alterations has been questioned3. Newer diffusion imaging sequences that employ multiple diffusion encoding schemes have been proposed to substitute the traditional ones -- which include the combination of oscillating gradient-echo (OGSE) and pulsed gradient spin-echo (PGSE) sequences and double diffusion encoding (DDE) sequence3, 4. The potential benefits of indices extracted with these sequences over the conventional ADC and FA have been reported in phantom studies and in a few pathological states.Neurocognitive performance, as assessed by a set of neuropsychological tests, is regarded as a standard measure to reflect brain functions such as attention and memory. Prior studies suggest the association between neurocognitive performance and imaging-derived tissue microstructural characteristics5, 6.
As an attempt to further identify the role of these newer diffusion imaging sequences, this study evaluated if the indices extracted with the OGSE, PGSE, and DDE sequences had any association with neurocognitive performance.
Methods
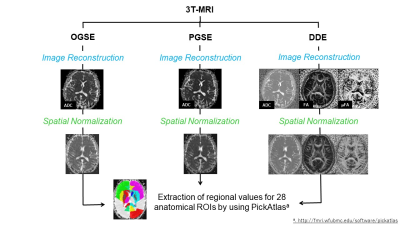
Thirty healthy subjects (17 men and 13 women; mean age = 28.87 ± 6.98 years) participated in this prospective study approved by the local institutional review board. Written informed consent was obtained from all participants.The participants underwent neurocognitive tests and MRI of the brain within a week interval. The former consisted of forward- and backward tapping (TST-F and TST-B) and digit span (DST-F and DST-B) tests, one (1-sec PASAT) and two-second (2-sec PASAT) paced auditory serial additional tests, trail making tests part-A (TMT-A) and B (TMT-B), and letter-number sequencing test. The latter was performed using a 3T-scanner (MAGNETOM Prisma, Siemens Healthineers, Erlangen, Germany) and a 64-channel head coil. Diffusion imaging was acquired using a prototype spin-echo EPI sequence and the following diffusion encoding variants: TR/TE=16700/113 ms, δ=4.8 ms, apparent Δ=4.6 ms, b=700 s mm-2, number of diffusion directions=4 for OGSE; TR/TE=16700/113 ms, δ=3.4 ms, Δ=92.7 ms, b=700 s mm-2, number of diffusion directions=4 for PGSE, and TR/TE=7000/84 ms, δ=12.2 ms, Δ=13.7 ms, b=800 s mm-2, number of diffusion directions=72 for DDE. The mean ADC, FA, and microscopic FA (μFA) values were extracted for 28 brain regions as defined by PickAtlas. The steps involved in image processing are summarized in Figure 1. Each regional value was then tested for correlation with neuropsychological test scores, by using Pearson's product-moment correlation analyses. Age and gender were taken as covariates. Statistical significance was set as P<0.005 considering multiple comparisons.
Results
The μFA and FA of the right caudate nucleus (r=0.516 and 0.512, P<0.005) and the μFA of the right temporal lobe (r=0.511, P<0.005) showed significant moderate positive correlations with the DST-F score (Figure 2). The ADC of the right sublobar white matter, extracted with the OGSE sequence, showed a moderate positive correlation with the TMT-B score (r=0.524, P<0.005) (Figure 3). The μFA of the right sublobar white matter (r=0.505, P=0.006) and the right occipital lobe (r=0.504, P=0.006) showed tendency toward correlation with the letter-number sequencing test score, as did the DDE sequence-derived ADC of the right thalamus with the DST-F score (r=-0.497, P=0.007) and the PGSE sequence-derived ADC of the right sublobar white matter with the TMT-B score (r=0.699, P=0.007)(Figure 4).Discussion
The caudate nucleus volume and functioning, the medial temporal cortical thickness, and superior temporal gyral lesions have been reported to affect the DST-F scores5, 6. Our observation of the correlation between the DST-F score and the μFA and FA of the right caudate nucleus and the μFA of the right temporal lobe may suggest the importance of their microstructural integrity in retaining short-term memory.The sublobar white matter contains several important association fiber tracts, such as corpus callosum, uncinate fasciculus, arcuate fasciculus, and inferior fronto-occipital fasciculus. The performance of TMT-B, a test which reflects central executive functioning, has been linked to the integrity of these structures7. Our observation of the correlation between the TMT-B score and the ADC of the right sublobar white matter, extracted with the OGSE sequence, is thought to suggest the role of sublobar white matter in conducting executive functions.
Right lateralization of brain regions responsible for neurocognition was observed, which is consistent with the previous observations8. According to literature, the left hemisphere is more specialized in language processing, whereas the right hemisphere is more specialized in attention, musical abilities, visuospatial tasks, and many aspects of emotion.
Larger number of correlations of the μFA with neurocognitive test scores suggests its superior sensitivity over the other indices. Our study further highlights the superiority of ADC extracted with OGSE over PGSE in identifying the imaging correlates of neurocognition, which may be due to differential sensitivity of these sequences to characterize tissue microstructure3.
Conclusions
The μFA and FA extracted from the DDE sequence and the ADC extracted from the OGSE sequence are reflective of the DST-F and TMT-B scores, and may serve as imaging alternatives of these neuropsychological tests in incorporative and ill patients.Acknowledgements
This study was supported by (i) the Global Institution for Collaborative Research and Education, Hokkaido University, Japan and (ii) the Grant-in-Aid for scientific research by the Japan Society for Promotion of Science (19K11317). The authors would like to thank Dr. Thorsten Feiweier from Siemens Healthcare for providing the diffusion sequence used in this work.References
- Tha KK, Terae S, Sugiura M, et al. Diffusion-weighted magnetic resonance imaging in early stage of 5-fluorouracil-induced leukoencephalopathy. Acta Neurol Scand 2002;106: 379-386.
- Tha KK, Terae S, Nakagawa S, et al. Impaired integrity of the brain parenchyma in non-geriatric patients with major depressive disorder revealed by diffusion tensor imaging. Psychiatry Res 2013; 212: 208 -215.
- Drobnjak I, Zhang H, Ianuş A, et al. PGSE, OGSE, and sensitivity to axon diameter in diffusion MRI: Insight from a simulation study. Magn Reson Med 2016; 75: 688-700.
- Shemesh N, Jespersen SN, Alexander DC, et al. Conventions and nomenclature for double diffusion encoding NMR and MRI. Magn Reson Med 2016; 75: 82-87.
- Aumont É, Arguin M, Bohbot V, West GL. Increased flanker task and forward digit span performance in caudate-nucleus dependent response strategies.Brain Cogn 2019;135:103576.
- Das SR, Mancuso L, Olson IR, Arnold SE, Wolk DA. Short-Term Memory Depends on Dissociable Medial Temporal Lobe Regions in Amnestic Mild Cognitive Impairment. Cereb Cortex 2016; 26: 2006-17.
- MacPherson SE, Cox SR, Dickie DA, et al. Processing speed and the relationship between Trail Making Test-B performance, cortical thinning and white matter microstructure in older adults. Cortex 2017; 95: 92–103.
- Liu H, Zhang L, Xi Q, Zhao X, Wang F, Wang X, Men W, Lin Q. Changes in Brain Lateralization in Patients with Mild Cognitive Impairment and Alzheimer's Disease: A Resting-State Functional Magnetic Resonance Study from Alzheimer's Disease Neuroimaging Initiative. Front Neurol 2018;9:3.
Figures
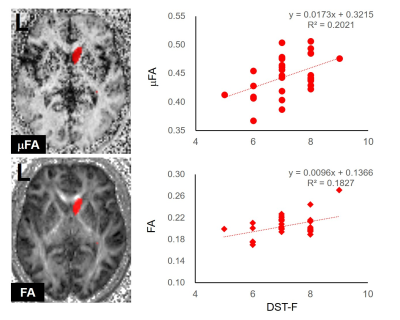
The region-of-interest (ROI) for the right caudate nucleus (Left). The ROI is overlaid on the microscopic fractional anisotropy (μFA) and fractional anisotropy (FA) maps of a subject.
Scatterplots showing the correlation between the μFA and FA of the right caudate nucleus and the forward digit span test (DST-F) scores (Right).
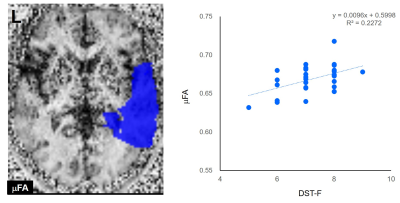
The ROI for the right temporal lobe (Left). The ROI is overlaid on the μFA map of a subject.
Scatterplots showing the correlation between the μFA of the right temporal lobe and the DST-F scores (Right).
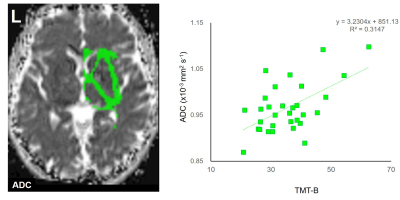
The ROI for the right sublobar white matter. The ROI is overlaid on an apparent diffusion coefficient (ADC) map extracted with the oscillating gradient spin-echo (OGSE) sequence (Left).
Scatterplots showing the correlation between the ADC, extracted with the OGSE sequence, of the right sublobar white matter and the trail-making test Part B (TMT-B) scores (Right).