1815
Quantitative susceptibility mapping reveals increased iron content of subcortical nuclei in hypertensive patients1XI’AN DAXING HOSPITAL, ShaanXi, Xi’an, China, 2Siemens Healthcare Ltd., ShaanXi, Xi’an, China, 3Siemens Healthcare Ltd., Beijing, China
Synopsis
Hypertension is known to be a major risk factor for damage to target organs, including the brain. Hypertension may be associated with increased heme content and non-heme iron contents. QSM is a potent imaging method that can provide a noninvasive and accurate measurement of iron content in the brain for diagnosing diseases and monitoring progression and treatment. This study evaluated the feasibility of QSM in the measurement of brain iron deposition in patients with hypertension. We found increased iron accumulation primarily in the deep gray matter nucleus in hypertensive patients.
Introduction
Hypertension is a leading cause of global disease and overall health loss1.Excluding age, hypertension is one of the most important risk factors for stroke and dementia. Regional excessive iron overload contributes to the continuous generation of radical species and toxic free radicals, which are harmful to the motor and cognitive functions of the brain, because they interfere with dopamine synthesis. Previous studies have detected abnormal serum ferritin levels in patients with hypertension. Hypertension may be associated with increased heme and non-heme iron contents beyond the normal effects of aging. Quantitative susceptibility mapping (QSM) can accurately reflect the spatial distribution of the tissue’s magnetic susceptibility, providing an indirect way of quantifying iron content in the brain. Most QSM studies have focused on tumors and neurodegenerative diseases, such as multiple sclerosis, Alzheimer’s Disease, and Parkinson’s Disease. However, to date, there have been few studies that use QSM to investigate the iron content of brain tissue in patients with hypertension. This study used QSM to examine the brain iron concentration in hypertensive patients and evaluate its association with the severity of hypertension.Methods
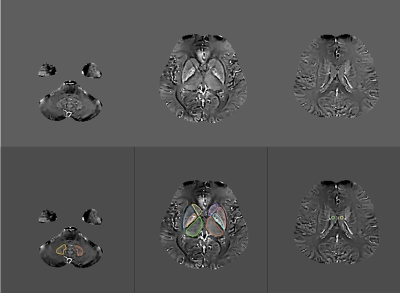
We collected two groups of age- and sex-matched participants: 24 patients with hypertension (HP, 12 males, 12 females; mean age = 65.3 ± 10.8 years) and 24 healthy controls (HC, 11 males, 13 females; mean age = 64.9 ± 10.5 years). All subjects were scanned with a 1.5T MR scanner (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany) using a 16-channel head coil. A multi-echo gradient echo (ME-GRE) sequence was applied for QSM acquisition with the following parameters: repetition time (TR) = 51 ms, TE1/ΔTE/TE8 = 8.22 ms/5 ms/43.22 ms, bipolar readout, slice thickness = 2 mm, flip angle = 20°, FOV = 230 mm × 210 mm, matrix size = 224 × 180, bandwidth = 200 Hz/Px, and slices = 56. The magnitude and phase images from ME-GRE acquisition were post-processed to generate susceptibility maps using the MEDI toolbox on Matlab (R2016b, Mathworks, Natick, MA, USA). The regions of interest at the bilateral dental nucleus (DN), dorsal thalamus (TH), putamen (PU), globus pallidus (GP), caudate (CA), and cerebrospinal fluid (CSF) within the lateral ventricle were manually drawn on the susceptibility maps to extract tissue susceptibility values (Fig.1). The susceptibility values of each structure were calculated as the average of the bilateral sides. Statistical analysis was performed using SPSS software (version 19.0, IBM Corp., Armonk, NY, USA). The measurement data were expressed as mean ± standard deviation (SD).p< 0.05 was considered statistically significant. Interobserver agreement was assessed by calculating the Cohen kappa statistic (kappa < 0.00: poor agreement, kappa= 0.00 - 0.20: slight agreement, kappa= 0.21 - 0.40: fair agreement, kappa= 0.40 - 0.60: moderate agreement, kappa= 0.61 - 0.80: substantial agreement; kappa= 0.81 - 1.00: almost complete agreement).Results
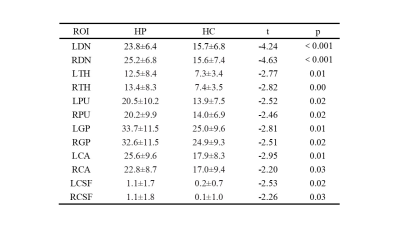
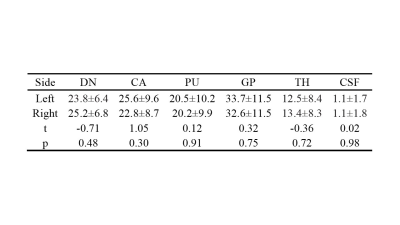
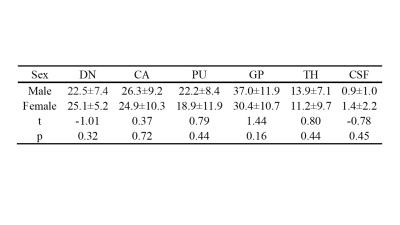
There were no significant differences in sex or age between the two groups. The susceptibility values of the bilateral DN, TH, PU, GP, CA, and CSF of HP were higher than those of the HC (p< 0.05) (Table 1). The magnetic susceptibility of HP showed no statistically significant difference in bilateral DN, TH, PU, GP, CA, and CSF, as well as in different sexes (p> 0.05) (Table 2,3). The interobserver agreement was high in both sequences (kappa= 0.67).Discussion
This study found that the magnetic susceptibility of subcortical structures of hypertensive patients, such as bilateral DN, TH, PU, GP, CA, and CSF, were higher than in healthy controls. The results suggested that the amount of iron deposition in the brains of hypertensive patients were overloaded. This iron overload might be caused by several factors. Cardiovascular and cerebrovascular diseases are the most common complications in patients with hypertension, including micro-bleeding, subcortical lacunar infarctions, and diffuse areas of white matter lesions. Hypertension-associated cerebral microbleeds are typically located in the basal ganglia and thalamus2. This may suggest that iron deposition in the hypertensive brain may be related to micro-bleeding. It is well established that QSM can be a noninvasive in vivo quantitative measure for tissue iron. Previous studies using phase imaging or susceptibility-weighted imaging found that abnormal iron deposition in the brain occurred in some neuropsychiatric diseases3. Compared with phase imaging, the QSM method provides more accurate measurements of the deposited iron.Conclusion
We used, for the first time, QSM to explore iron accumulation in subcortical nuclei in the setting of hypertension. Our results indicated the role of excess brain iron in the deep gray matter in hypertension and suggested that iron may be a potential biomarker for further understanding the pathophysiological mechanism of hypertension.Acknowledgements
We thank Shaoyu Wang, Xiang Feng of Siemens Healthcare, Ltd., Xi’an, China, for technical support.References
1.Zhua Y, Chen G, Bo Y, Liu Y. Markers of iron status, blood pressure and incident hypertension among Chinese adults. Nutrition, metabolism, and cardiovascular diseases : NMCD 2019; 29(8): 830-836.
2.Meissner A. Hypertension and the Brain: A Risk Factor for More Than Heart Disease. Cerebrovasc Dis 2016; 42(3-4): 255-262.
3.Chen Q, Chen Y, Zhang Y, et al. Iron deposition in Parkinson’s disease by quantitative susceptibility mapping. BMC Neuroscience 2019; 20(1): 23.
Figures