1814
Highly Accelerated Wave-CAIPI Post-Contrast 3D-T1 Compared to Standard Post-Contrast 3D-T1 SPACE for Detection of Abnormal Enhancing Lesions.1Radiology, Massachusetts General Hospital, Boston, MA, United States, 2Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 3Siemens Healthineers, Erlangen, Germany, 4Siemens Shenzhen Magnetic Resonance Ltd., Shenzhen, China, 5Harvard Medical School, Boston, MA, United States
Synopsis
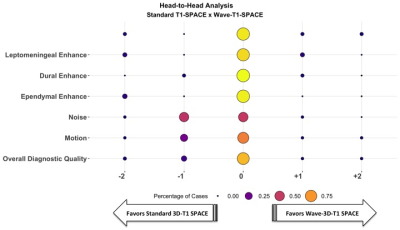
We present the first large-scale evaluation of the diagnostic performance and image quality of highly accelerated Wave-CAIPI post-contrast 3D-T1 SPACE (Wave-T1 SPACE) compared to standard post-contrast 3D-T1 SPACE for the detection of intracranial enhancing lesions in patients undergoing 3T MRI scanning. Two neuroradiologists assessed the images in a head-to-head comparison, and found no significant difference between the two sequences for detection of abnormal intracranial enhancement and overall diagnostic quality, despite a nearly 3-fold decrease in acquisition time of post-contrast Wave-T1 SPACE. The application of highly-accelerated 3D imaging may improve use of MRI resources while reducing motion artifacts and patient anxiety.
Purpose
To compare the diagnostic performance and image quality of highly accelerated Wave-CAIPI post-contrast 3D-T1 SPACE (Wave-T1 SPACE) to standard post-contrast 3D-T1 SPACE for the detection of intracranial enhancing lesions.Methods
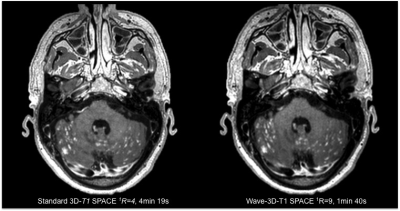
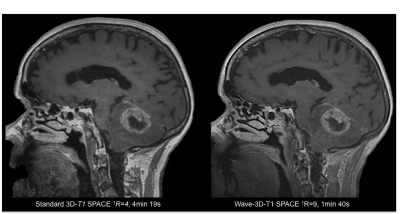
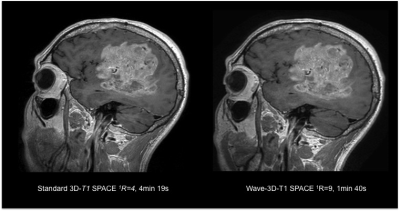
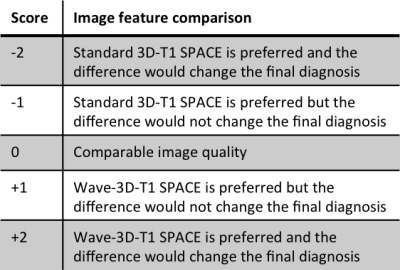
Sixty-five patients undergoing 3T clinical brain MRI scanning with and without contrast were prospectively enrolled. All MRI scans included a standard post-contrast 3D-T1 SPACE (R=4, acquisition time TA=4min 19s, 0.9 mm isotropic resolution) and resolution-matched post-contrast prototype Wave-T1 SPACE sequence (R=9, TA=1min 40s). The studies were performed on clinical 3T MRI scanners (MAGNETOM Prisma; Siemens Healthcare, Erlangen, Germany) with 20- or 32-channel multi-array receiver coils. The most common indications for MRI were the evaluation of primary intracranial tumors (N=26) and screening for metastatic disease (N=23). Two neuroradiologists blinded to sequence type performed a head-to-head comparison of the image. A predefined 5-point scale was used for grading of abnormal intracranial enhancement (dural, parenchymal, leptomeningeal, and ependymal), motion artifacts and noise, and overall diagnostic quality. A third reader adjudicated discrepancies.Results
The Wave-T1 SPACE sequence showed no significant difference in the visualization of abnormal intracranial enhancement compared to the standard sequence. Wave-T1 SPACE images demonstrated slightly higher image noise and a similar degree of motion artifacts in the majority of cases, with no impact on overall diagnostic quality. The balloon plot and the representative examples in the figures demonstrate the comparable diagnostic quality of the standard post-contrast T1 SPACE and Wave-T1 SPACE sequences in delineating pathological enhancement.Conclusion
The post-contrast 3D-Wave-T1 SPACE sequence allows an approximately 3-fold reduction in acquisition time with equivalent performance in identifying parenchymal, leptomeningeal, dural and ependymal enhancing lesions compared to a 4min 19s resolution-matched standard post-contraste T1 SPACE sequence. These results support the use of accelerated volumetric Wave-T1 SPACE over standard T1 SPACE in clinical brain MR protocols requiring contrast. This will decrease patient time on the scanner and the aggregated time savings may improve the utilization of MRI resources, while reducing the number of motion artifacts and nondiagnostic exams, with better patient comfort and reduced anxiety.Acknowledgements
No acknowledgement found.References
Bilgic B, Gagoski BA, Cauley SF, et al. Wave-CAIPI for highly accelerated 3D imaging. Magn Reson Med. 2015;73(6):2152-2162. doi:10.1002/mrm.25347
Cauley SF, Setsompop K, Bilgic B, Bhat H, Gagoski B, Wald LL. Autocalibrated wave-CAIPI reconstruction; Joint optimization of k-space trajectory and parallel imaging reconstruction. Magn Reson Med. 2017;78(3):1093-1099. doi:10.1002/mrm.26499
Figures