1803
Assessing gray and white matter glutamatergic turnover in human brain non-invasively using 1H MRS and deuterated glucose1Department of Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2Sidra Medicine, Doha, Qatar, 3LARC, Qatar University, Doha, Qatar, 4Department of Neurology, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Cerebral metabolism is reported to be monitored by various techniques including 13C/2H MR spectroscopy and 18F-FDG Positron emission tomography. It is well known that 1H MRS allows the non-invasive detection and quantification of neurochemicals. In this study, we performed 1H MRS in conjuction with the oral administration of [6,6′-2H2]glucose in a healthy volunteer at 3T and 7T to measure turnover of glutamate in gray and white matter. As 2H is invisible on 1H MRS, the 2H enrichment of glutamate leads to a corresponding drop in its 1H MR resonance measured via LCModel.
Introduction
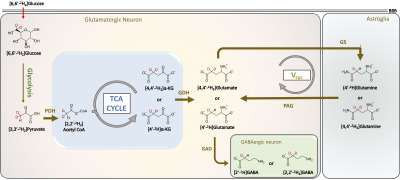
Multinuclear Magnetic Resonance Spectroscopy (MRS) in conjunction with the administration of labeled substrates such as glucose, pyruvate has provided great insights into cerebral energetics and neurotransmitter cycling1,2. Very recently, 2H MRS has been reported to offer metabolic information in a healthy brain and cancer pathology3,4. In this study, we present a novel strategy by combining the administration of [6,6′-2H2]glucose and 1H MRS as a tool to monitor cerebral energetics in humans at 3T and 7T MRI. [6,6′-2H2]Glucose is majorly taken up by neuronal cells in the brain, where it is metabolized to 2H-labeled pyruvate via glycolysis. The 2H label is further transferred to the TCA via acetyl-CoA enriching a-ketoglutarate and glutamate (Figure 1). The neurotransmitter pool of glutamate is transported to astroglial cells where it is converted into glutamine by glutamine synthetase (GS). Glutamine is transferred to neurons to replenish the neuronal glutamate pool by conversion of neuronal glutamine into glutamate by the action of phosphate-activated glutaminase (PAG) thereby completing the glutamate-glutamine neurotransmitter cycling. The use of non-toxic, non-radioactive [6,6′-2H2]glucose along with 1H MRS offers a unique opportunity to detect and quantify the kinetics of 2H label transfer into downstream metabolites via the signal reduction in the corresponding 1H spectrum with high spectral and temporal resolution. The detection of exchanged label turnover quantitative measurement (ELOQUENT) by 1H MRS (eMRS) offers a brilliant tool for measuring cellular energetics non-invasively. In this work, we demonstrate the application of eMRS and oral administration of [6,6′-2H2]glucose to humans as a promising novel alternative approach to multinuclear MRS studies to determine cerebral energetics in vivo.Methods
The protocol was approved by the Institutional Review Board. Written informed consent was obtained prior to the scans. This preliminary study was performed on 2 subjects (1 male 35 yr old, and 1 female 27 yr old). MR experiments were performed at 3T and 7T Siemens scanners using a 64-channel (3T) and 32-channel (7T) receive head coils. The healthy volunteers underwent overnight fasting before the MRS studies. The blood glucose levels of the subjects were measured Pre and Post MRS experiments. Immediately following the oral administration of [6,6′-2H2]glucose in drinking water, 1H MRS was performed at 3T. 1H MR spectra were acquired from two voxels positioned in the gray and white matter (30×15×10 mm3) using spin-echo sequence (TR/TE = 2000/30 ms, spectral width = 2 kHz, averages = 128, scan time = 5 min). Another spectrum with 4 averages was acquired without water suppression to obtain the water reference signal for quantification. After the passage of 80 minutes since [6,6′-2H2]glucose intake, 1H MR spectra were again acquired in the same voxels. The subject was then transferred to the 7T scanner and 1H MRS was performed on the same gray and white matter voxels at ~120 minutes post-[6,6′-2H2]glucose intake. The baseline 1H MR spectra from GM and WM at 7T were acquired 4 days post-[6,6′-2H2]glucose intake. Metabolites were quantified using LCModel software5. Once metabolite concentration at each time point was estimated, calculation of metabolite fractional enrichment (FE) was performed by subtracting post-infusion levels from pre-infusion levels. All FE enrichment plots report mean values with the standard error of the mean.Results and Discussion
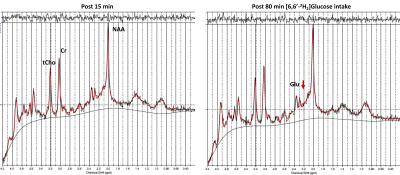
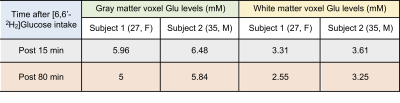
The comparison between 1H MR spectra 15 min post and 80 min post-[6,6′-2H2]glucose intake showed a clear reduction in glutamate resonance in both gray and white matter of one healthy volunteer (Figure 1). This indicated that the 2H label incorporation into Glu-H4 from [6,6′-2H2]glucose via glycolysis and TCA cycle led to glutamate signal reduction detected via 1H MRS. LCModel analysis revealed a reduction in the glutamate concentration in both volunteers at 80 minutes Post [6,6′-2H2]glucose intake due to turnover of 2H on Glu-H4 resonance via glycolysis and TCA cycle. Quantification of the FE (Table 2A) revealed 16% (Female, 27) and 10% (Male, 35) FE at 80 min Post glucose administration in the gray matter. Similar results were found in the white matter at 45 min post-infusion. The estimation of the rates of glutamatergic, GABAergic and glial TCA cycles in addition to neurotransmitter cycling (glutamatergic and GABAergic) via eMRS experiments are currently ongoing. We anticipate these results would compare well with the values already reported in the literature6-9. This approach is expected to enable a wide range of studies probing metabolic derangements in vivo across medical disciplines.Acknowledgements
This project was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institute of Health under award number P41EB015893.References
1. Shulman, R. G., & Rothman, D. L. 13C NMR of intermediary metabolism: implications for systemic physiology. Ann. Rev. Phys. 63, 15-48 (2001)
2. Henry, P. G., et al. In vivo 13C NMR spectroscopy and metabolic modeling in the brain: a practical perspective. Magn. Reson. Imaging, 24, 527-539 (2006)
3. Lu, M., Zhu, X. H., Zhang, Y., Mateescu, G. & Chen, W. Quantitative assessment of brain glucose metabolic rates using in vivo deuterium magnetic resonance spectroscopy. J. Cereb. Blood Flow Metab. 37, 3518-3530 (2017)
4. De Feyter, H. M. et al. Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo. Sci. Adv. 4, eaat7314 (2018)
5. Provencher, S. W. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 14, 260-264 (2001)
6. de Graaf, R. A., Mason, G. F., Patel, A. B., Behar, K. L. & Rothman, D. L. In vivo 1H-[13C]-NMR spectroscopy of cerebral metabolism. NMR Biomed. 16, 339-357 (2003)
7. van Eijsden, P., Behar, K. L., Mason, G. F., Braun, K. P., & De Graaf, R. A. In vivo neurochemical profiling of rat brain by 1H‐[13C] NMR spectroscopy: cerebral energetics and glutamatergic/GABAergic neurotransmission. J. Neurochem. 112, 24-33 (2010)
8. Wijnen JP et al. In vivo 13C magnetic resonance spectroscopy of a human brain tumor after application of 13C-1-enriched glucose. Magn. Res. Imaging. 28, 690-7 (2001)
9. Bomezbaur F et al. Altered Brain Mitochondrial Metabolism in Healthy Aging as Assessed by in vivo Magnetic Resonance Spectroscopy. J. Cereb. Blood. Flow. Metab. 30, 211-221 (2010)
Figures