1802
Comparing aMRI to DENSE for the assessment of brain tissue motion1Radiology, University Medical Center Utrecht, Utrecht, Netherlands, 2Radiology, Stanford University, Stanford, CA, United States, 3School of Medicine, Queen’s University, Kingston, ON, Canada, 4Anatomy and Medical Imaging, University of Auckland, Auckland, New Zealand, 5University Medical Center Utrecht, Utrecht, Netherlands
Synopsis
aMRI holds great potential for assessing brain motion/strain using images acquired from readily-available sequences. However, registration is needed to extract displacements from the motion-amplified images, which may limit its accuracy. In this study we separately assessed the errors induced by registration limitations and by imperfections in the aMRI amplification. Displacements were extracted from aMRI and DENSE-amplified images using a common registration algorithm, which were then compared to a ground truth. Although significant differences were found between DENSE-amplified images and aMRI, the aMRI-derived displacements were comparable to the ground truth, strengthening the potential of aMRI for investigating brain motion in disease.
Introduction
Brain tissue exhibits cardiac-induced pulsatile motion which may be altered in diseases that affect intracranial pressure1, providing a potential means to better quantify and understand these diseases. Both DENSE2 and amplified MRI3,4 (aMRI) have the sensitivity to discern the subtle displacements that are associated with brain tissue motion. aMRI estimates displacements through a post-processing algorithm applied on conventional cine-images, which yields a great potential for assessing brain motion on clinically available scanners with readily available sequences. Furthermore, brain tissue strain is derivable from tissue displacement fields, which could provide insights into diseases that affect the (visco)elastic properties of brain tissue, or the cerebral small vessels which act as a conduit for cardiac-induced tissue strain. However, a registration algorithm is needed to extract displacements from the amplified motion images generated by aMRI, which may limit its accuracy. Moreover, accurate quantification of strain critically depends on good estimation of the amplification factor. In this study we aim to separately assess the errors induced both by registration limitations and by potential imperfections in the amplification of aMRI. This is done by comparing both DENSE-amplified images and aMRI-derived brain tissue displacements to a known ground truth.Methods
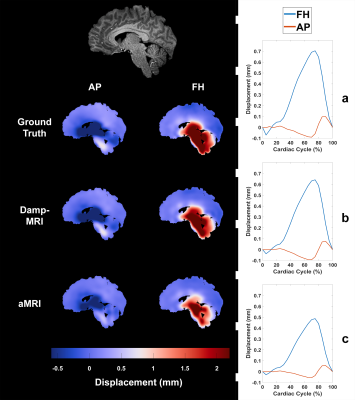
Anterior-Posterior (AP) and Feet-Head (FH) 4D DENSE images were acquired and processed from 8 subjects as previously described5 to extract brain tissue displacements covering the entire cardiac cycle (0.93x0.93x1 mm3 interpolated resolution). A high-resolution T1-weighted image (resolution = 0.93x0.93x1 mm3) was also acquired for each subject. Ground truth displacements (GTD) were created by selecting a mid-sagittal slice of the T1 image (10-20 mm left/right from the interhemispheric fissure) and then spatially smoothing the AP and FH DENSE displacements associated with that mid-sagittal slice with a Gaussian filter (kernel=21x21 pixels, sigma=3.5). The mid-sagittal T1-weighted slice was then deformed by each cardiac phase of the GTD to create DENSE-animated images (Dani-MRI). Phase-based amplification4 was used to 10x amplify the Dani-MRI to create aMRI. As a reference, DENSE-amplified images (Damp-MRI) were similarly created by linearly scaling the GTD by a factor of 10 and then using the scaled GTD to deform the mid-sagittal T1 image. Cardiac-induced brain tissue displacements were extracted from the Damp-MRI and aMRI through registration with elastix6. Linear regression was then used to investigate the amplification factor (slope) and agreement (R2) between Damp-MRI derived displacements and the GTD, and also between aMRI-derived displacements and the GTD. Student’s T-test was used to assess differences between the Damp-MRI and aMRI regressions. Note that mismatch between Damp-MRI and GTD selectively reflects errors from the registration, while aMRI vs. GTD reflects both registration errors and imperfections of the amplification algorithm used by aMRI.Results
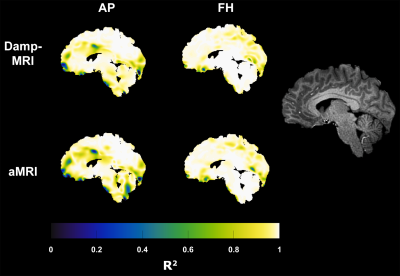
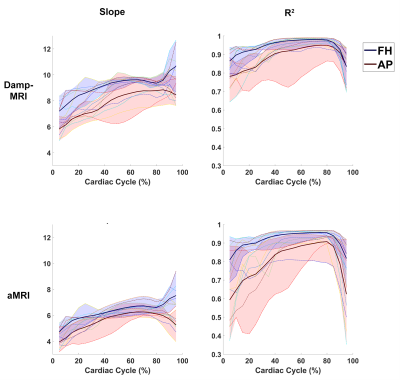
Figure 1 shows example displacement maps and curves for the GTD as well as Damp-MRI and aMRI-derived displacements. The group mean slopes (at peak displacement within brain tissue mask) of the Damp-MRI derived displacements versus the GTD were (mean±std) 8.2±0.8 and 8.8±0.2 for AP and FH displacements, respectively. The group mean slopes for the aMRI-derived displacements versus the GTD were 5.8±0.3 and 6.2±0.4 for AP and FH displacements, respectively. The aMRI slopes were significantly lower than the Damp-MRI slopes for both AP (p<0.0005) and FH (p<0.0005) displacements. The spatial R2 agreement between the GTD, Damp-MRI and aMRI derived displacements is shown in figure 2, whilst figure 3 shows the temporal stability over the cardiac cycle of the agreement and amplification factor. The amplification is noticeably biased, for both Damp-MRI and aMRI. The AP displacements were least well captured (lowest agreement and amplification), which may partly be due to the generally lower amplitudes of AP motion compared to FH motion.Discussion
We investigated the accuracy of Damp-MRI and aMRI-derived displacements in an ideal case without image artefacts, using a known amplification factor and known displacements. Both Damp-MRI and aMRI derived displacements showed generally good agreement to the GTD. The estimation of the amplification factor (slope) from Damp-MRI displacements was significantly more accurate than those from aMRI, suggesting the need for further calibration of the amplification parameters. Poorer agreement of derived displacements in the later phases of the cardiac cycle was found for both Damp-MRI and aMRI. As such, these are likely related to limitations of the registration algorithm and underscores the importance of the registration algorithm used to extract displacements, and the need for artefact-free images which could otherwise misguide the registration. Of note, accurate strain computations would be compounded by the different amplification factors found in this study for the AP and FH displacements. Future work is warranted to investigate the accuracy of brain tissue strain obtained from aMRI derived displacements, and to utilise these measurements to explore changes to brain tissue with disease.Conclusion
aMRI-derived displacements are comparable to DENSE in the ideal case, strengthening the potential of aMRI as a means for investigating brain tissue displacements. However, there was limited success in extracting the amplification factor from the derived displacements, which is partly attributable to limitations of the required registration step, and partly to imperfections of the amplification algorithm itself. Future work is necessary to investigate to what extent these limitations hinder practical use of aMRI for studying brain motion in health and disease.Acknowledgements
No acknowledgement found.References
1. Saindane AM, Qiu D, Oshinski JN, et al. Noninvasive Assessment of Intracranial Pressure Status in Idiopathic Intracranial Hypertension Using Displacement Encoding with Stimulated Echoes (DENSE) MRI: A Prospective Patient Study with Contemporaneous CSF Pressure Correlation. Am J Neuroradiol. 2018;39(2):311-316. doi:10.3174/ajnr.A5486
2. Adams AL, Kuijf HJ, Viergever MA, Luijten PR, Zwanenburg JJM. Quantifying cardiac-induced brain tissue expansion using DENSE. NMR Biomed. 2019;32(2):1-13. doi:10.1002/nbm.4050
3. Holdsworth SJ, Rahimi MS, Ni WW, Zaharchuk G, Moseley ME. Amplified magnetic resonance imaging (aMRI). Magn Reson Med. 2016;75(6):2245-2254. doi:10.1002/mrm.26142
4. Terem I, Ni WW, Goubran M, et al. Revealing sub-voxel motions of brain tissue using phase-based amplified MRI (aMRI). Magn Reson Med. 2018;80(6):2549-2559. doi:10.1002/mrm.27236
5. Adams AL, Sloots J-J, Luijten PR, Zwanenburg JJM. SNR analysis of retrospectively gated DENSE at 7T for the measurement of brain tissue pulsatility. In: Proceedings of the Joint Annual Meeting ISMRM-ESMRMB. Paris, France; 2018.
6. Metz CT, Klein S, Schaap M, van Walsum T, Niessen WJ. Nonrigid registration of dynamic medical imaging data using nD+t B-splines and a groupwise optimization approach. Med Image Anal. 2011;15(2):238-249. doi:10.1016/j.media.2010.10.003
Figures