1800
Ultrashort-T2* component differences in white matter regions of the brain1Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States
Synopsis
This work presents a study detailing how ultrashort-T2* measurements can provide new and complimentary characterizations and differentiations of white matter anatomy in comparison to classic DTI measurements. Specifically the magnitude and frequency shift components of the measured signal are significantly different between white matter with thick versus thin myelin sheaths and provides new methods of assessing myelin content in white matter when compared to FA, MD, and V1 values. These results show that this method could be used to better characterize and study demyelinating diseases such as multiple sclerosis.
Introduction
Myelin plays a critical role in facilitating rapid neural signal conduction across the brain. The degree of myelination and its structure are key features that can characterize many neurological disorders including demyelinating diseases such as multiple sclerosis and neurodegenerative diseases such as Alzheimer’s disease.MRI is the preferred imaging modality for assessing myelin given its sensitivity to water content, binding, and diffusion. Recent studies have characterized ultrashort-T2* components in the brain using a novel ultrashort echo time (UTE) relaxometry method. These components are most likely associated with methylene protons in myelin phospholipid membranes and are a potentially more direct measure of myelination1, 2, 3, 4, 5.
To characterize this method in white matter, we compared ultrashort-T2* measurements derived from a fitted signal model in various white matter structures in the brain. Overall our results show that ultrashort-T2* signal magnitude and frequency shift are significantly different in white matter structures which have thicker myelin sheaths. Additionally, we present DTI data on the same datasets showing that ultrashort-T2* measurements provide complimentary characterizations and differentiations of white matter anatomy when compared to DTI measurements.
Methods
Whole brain relaxometry was performed using a 3D radial pulse sequence supporting UTEs with a nonselective, hard pulse excitation. The delay between excitation and readout was shifted between TRs to acquire a set of 20 different TEs within a single scan ranging from 24 µs to 3.5 ms. Additionally, DTI images were taken with the following settings: B = 1000, DIR = 30 and B = 3000, DIR = 64. Other key parameters were 2-2.2 mm isotropic resolution, 4.1-5.8 fold parallel imaging acceleration reconstructed with non-Cartesian ESPIRiT6.All sets of TE data were fit to the following signal model to estimate the signal of each component, relaxation times, and frequency shifts:
$$S(\vec{r}, TE) =\sum_{k=1}^{N} \hat{S}_{0, k}(\vec{r})exp(-TE/T^{*}_{2, k}(\vec{r}))exp(i(2\pi \delta f_{k}(\vec{r})TE + \phi _{k}(\vec{r})))$$
with N=2 components where the first captured all long-T2* (> 1 ms) components and the second captured the ultrashort-T2* (< 1 ms) components. Parameter maps representing each of these measurements were generated in MATLAB (Mathworks).
Fractional Anisotropy (FA), Mean Diffusivity (MD), V1, V2, V3, L1, L2, L3, and ADC maps were generated with the FDT function in FSL. Included atlases “JHU white-matter tracts", "JHU white-matter-label," and “HarvardOxford Subcortical” were registered to UTE image space for each dataset using the FLIRT function in FSL7,8. ROI based analysis was performed using the fslroi function in FSL and a custom MATLAB script. Ultrashort-T2* and DTI values were averaged in specific ROIs across 8 healthy volunteers. The Wilcoxon rank sum test was performed in MATLAB to test for significant ultrashort-T2* differences between white matter tracts, deep gray matter structures, and overall cortical gray matter.
Results and Discussion
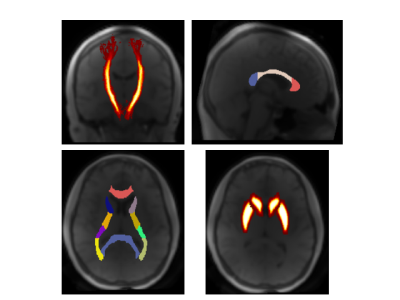
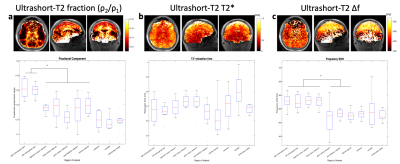
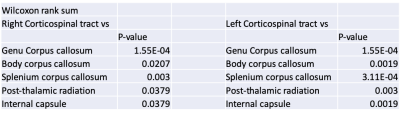
From figures 2 and 3 we observe the ultrashort-T2* fractional component in both corticospinal tracts is approximately 0.16 which is significantly higher than other white matter structures including the corpus callosum, post-thalamic radiation, and internal capsule. The corticospinal tract consists of a wide variety of neurons consisting of axons from the motor cortex, premotor cortex, somatosensory cortex, parietal lobe, and cingulate gyrus9. We hypothesize that this result is due to the structure of myelin in our chosen ROI, where the structure acts as an “information highway” connecting axons from many areas of the brain involved in voluntary movement.Figure 2 also shows frequency shift was significantly lower in the corticospinal tracts as well as the body and splenium corpus callosum, structures known to have higher myelination compared with other white matter structures. Given these measurements, we hypothesize that the frequency shift parameter could be correlated with myelin thickness in a given ROI.
No significant differences in ultrashort-T2* relaxation time were observed in any of the white matter tracts analyzed. Average ultrashort-T2* relaxation times for all regions were measured between 0.6 and 0.7 ms. This result could be a product of the fitting model relaxation term reflecting only the presence of myelin bound protons and the measure may not be dependent on the relative abundance of this component in a given white matter region.
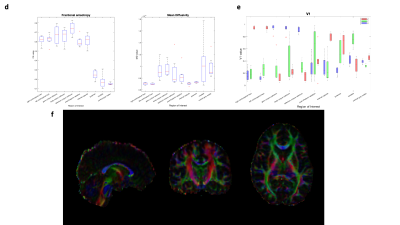
DTI is useful for determining water diffusion directionality in a given ROI and is robust at separating white and gray matter. For example measured FA is significantly lower in deep gray matter structures and cortical gray matter (Figure 4). While such information is useful in determining the presence of free water protons surrounding and within myelin membranes, it does not give information about myelin itself directly. Alternatively, given the results from these studies, we feel our ultrashort-T2* measurements can effectively differentiate between white matter structures, and can better characterize their anatomy. Such techniques could be more useful in characterizing structure and function of demyelinating diseases such as multiple sclerosis.
Conclusion
This work presents a study detailing how ultrashort-T2* measurements can provide new characterizations and differentiations of white matter anatomy. Specifically, the magnitude and frequency shift components of the signal are significantly different between white matter with thick versus thin myelin sheaths and provides new methods of assessing myelin content in white matter. These results show that this method could be used to better characterize and study demyelinating diseases.Acknowledgements
This work was supported by research grants: NIH R21NS089004, NMSS research grant PP3360.References
1. Horch, R. A., Gore, J. C., & Does, M. D. (2011) Origins of the ultrashort-T(2) (1) H NMR signals in myelinated nerve: A direct measure of myelin content?. Magn Reson Med 66, 24-31.
2. Wilhelm, M. J., Ong, H. H., Wehrli, S. L., Li, C., Tsai, P.-H., Hackney, D. B., & Wehrli, F. W. (2012) Direct magnetic resonance detection of myelin and prospects for quantitative imaging of myelin density. Proc Natl Acad Sci U S A 109, 9605-10.
3. Du, J., Ma, G., Li, S., Carl, M., Szeverenyi, N. M., VandenBerg, S., Corey-Bloom, J., & Bydder, G. M. (2014) Ultrashort echo time (UTE) magnetic resonance imaging of the short T2 components in white matter of the brain using a clinical 3T scanner. Neuroimage 87, 32-41.
4. Du J ,Sheth V ,He Q ,Carl M ,Chen J ,Corey-Bloom J ,Bydder GM. Measurement of T1 of the ultrashort T2* components in white matterof the brain at 3T. PLoS One 2014;9:e103296.
5. Boucneau, T., Cao P, Tang, S., Han M., Xuan D., Henry RG., & Larson PEZ. (2018) In Vivo Characterization of Brain Ultrashort-T2 Components. Magn Reson Med, 80(2), 726-735.
6. Uecker, M., Lai, P., Murphy, M. J., Virtue, P., Elad, M., Pauly, J. M., Vasanawala, S. S., & Lustig, M. (2013) ESPIRiT-an eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magn Reson Med , 71(3), 990-1001.
7. M. Jenkinson and S.M. Smith. A global optimisation method for robust affine registration of brain images. Medical Image Analysis, 5(2):143-156, 2001.
8. M. Jenkinson, P.R. Bannister, J.M. Brady, and S.M. Smith. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17(2):825-841, 2002.
9. Hall, Arthur C. Guyton, John E. (2005). Textbook of medical physiology (11th ed.). Philadelphia: W.B. Saunders. pp. 687–690.
Figures