1794
Resting brain entropy in the default mode network and the executive network may serve as a functional brain reserve1Radiology, University of Maryland School of Medicine, Baltimore, MD, United States
Synopsis
Human brain relies on long-range coherent activity to execute complex function. The long-range coherence can be measured by brain entropy mapping, which has gained increasing research interest in recent years. The purpose of this study is to examine the relationship between brain entropy and brain functions using large data.
Introduction
The long-range temporal coherence (LRTC) of brain activity is fundamental to high-order brain functions [1-4]. LRTC can be measured by entropy, which indicates the irregularity or incoherence. Derived from resting state fMRI (rsfMRI), functional brain entropy (fBEN) has been shown to be reproducible across time and sensitive to various brain diseases and to focal neuromodulations [5-8]. An open but fundamental question is how fBEN is related to high-order brain functions or behavior. The purpose of this study was to address this question using large data from the human connectome project (HCP) [9]Materials and Methods
rsfMRI data, demographic data, and behavior data from 860 healthy young subjects (age: 22-37 yrs) were downloaded from HCP. Each subject had 4 resting scans acquired with the same multi-band sequence[10] but differed by the readout directions: readout was from left to right (LR) for the 1st and 3rd scan and right to left (RL) for the other 2 scans. The pre-processed rsfMRI data in the MNI space were downloaded from HCP and were smoothed with a Gaussian filter with full-width-at-half-maximum (FWHM)=6mm to suppress the residual inter-subject brain structural difference after brain normalization and artifacts in rsfMRI data introduced by brain normalization. fBEN mapping was performed with BENtbx using the default settings [5]. 4 graphic processing unit (GPU) video cards and CUDA (the parallel computing programming platform created by Nvidia Inc) were used to accelerate fBEN computing. Mean fBEN maps of the first LR and RL scans and the second LR and RL scans were calculated for the following analyses. Age, sex, and education associations of resting fBEN were assessed with simple regression using SPM. Associations of fBEN to fluid intelligence (measured by the Penn Matrix Test [11]), and functional task performance were similarly examined but with age and sex included as nuisance covariates. Task performance was measured by the accuracy of button selection during the off-magnetic behavioral tasks. The significance level of the analysis results was p<0.05. Multiple comparison correction was performed with the family wise error theory [12].Results and Discussion
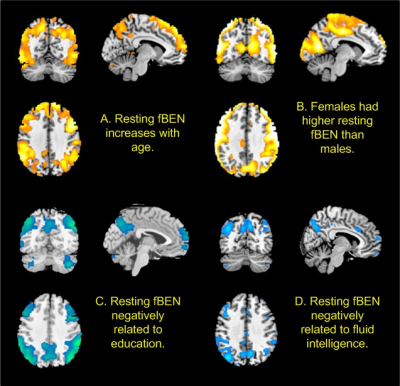
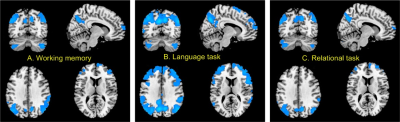
The GPU-based implementation of BENtbx gained 10-fold computation speed acceleration though it still took roughly a month to calculate fBEN maps for all 1023 subjects (860 had complete data). Based on the first mean fBEN maps (the mean of the first LR and RL scans), Fig. 1 shows the association maps of fBEN to age, sex, education years, and fluid intelligence. Very similar results were found in the second (retest) fBEN maps. fBEN increases with age (Fig. 1A) in the prefrontal executive network (ECN) and the frontal-temporal-parietal default mode network (DMN), suggesting that aging might affect those areas more as demonstrated by other neuroimaging studies[13]. Females showed higher fBEN in visual cortex, motor area, and some part of precuneus (Fig. 1B), which is consistent with previous studies [14]. Longer education years were associated with decreased fBEN in ECN and DMN (Fig. 1C). In Fig 1D, fluid intelligence was associated with lower fBEN in part of ECN and DMN. Education years is a major indicator of cognitive reserve [15] for compensating the aging-related behavioral impairments. The findings of longer education suppressing the age-related fBEN increase in DMN and ECN suggest that lower fBEN in DMN and ECN may be a functional representation of the cognitive reserve, that is to say, a functional reserve (FUR). Fig 2 shows the associations of resting fBEN to functional task performance. fBEN in DMN and part of ECN was negatively correlated with different task performance. These negative associations further support the potential role of the long-range coherent resting activity in DMN and ECN as a FUR for brain function.Conclusion
We found age-related fBEN increase in ECN and DMN, which decreases with education years. Lower fBEN in ECN/DMN correlates to better fluid intelligence and functional task performance. These findings suggest a potential FUR role of the coherent resting state brain activity which can be improved by education and may result in better brain function.Acknowledgements
This work was supported by NIH/NIA grant: 1 R01 AG060054-01A1.References
1. Buzsáki, G. and A. Draguhn, Neuronal oscillations in cortical networks. science, 2004. 304(5679): p. 1926-1929.
2. Saleh, M., et al., Fast and slow oscillations in human primary motor cortex predict oncoming behaviorally relevant cues. Neuron, 2010. 65(4): p. 461-471. 3. Thut, G., C. Miniussi, and J. Gross, The functional importance of rhythmic activity in the brain. Current Biology, 2012. 22(16): p. R658-R663. 4. Gregoriou, G.G., et al., High-frequency, long-range coupling between prefrontal and visual cortex during attention. science, 2009. 324(5931): p. 1207-1210.
5. Wang, Z., et al., Brain Entropy Mapping Using fMRI. PloS One, 2014. 9(3): p. e89948.
6. Zhou, F., et al., Resting State Brain Entropy Alterations in Relapsing Remitting Multiple Sclerosis. PLoS One, 2016. 11(1): p. e0146080.
7. Xue, S.W., et al., Resting-state brain entropy in schizophrenia. Compr Psychiatry, 2019. 89: p. 16-21.
8. Song, D., et al., Reduced brain entropy by repetitive transcranial magnetic stimulation on the left dorsolateral prefrontal cortex in healthy young adults. Brain imaging and behavior, 2018: p. 1-9.
9. Van Essen, D.C., et al., The WU-Minn Human Connectome Project: an overview. Neuroimage, 2013. 80: p. 62-79.
10. Moeller, S., et al., Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med, 2010. 63(5): p. 1144-53.
11. Bilker, W.B., et al., Development of abbreviated nine-item forms of the Raven’s standard progressive matrices test. Assessment, 2012. 19(3): p. 354-369.
12. Nichols, T.E. and S. Hayasaka, Controlling the Familywise Error Rate in Functional Neuroimaging: A Comparative Review. Statistical Methods in Medical Research, 2003. 12: p. 419-446. 13. Drachman, D.A., Aging of the brain, entropy, and Alzheimer disease. Neurology, 2006. 67(8): p. 1340-52.
14. Li, Z., et al., Hyper-resting brain entropy within chronic smokers and its moderation by Sex. Sci Rep, 2016. 6: p. 29435.
15. Stern, Y., Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord, 2006. 20(2): p. 112-7.
Figures