1793
White matter neuroplasticity: Motor learning modifies hemodynamic responses in the internal capsule1Cumming School of Medicine, University of Calgary, Calgary, AB, Canada, 2Surrey Memorial Hospital ImageTech Laboratory, Fraser Health, Surrey, BC, Canada, 3Biomedical Physiology and Kinesiology, Simon Fraser University, Burnaby, BC, Canada, 4Faculty of Applied Sciences, Simon Fraser University, Burnaby, BC, Canada, 5Djavad Mowafaghian Centre for Brain Health, University of British Columbia, Vancouver, BC, Canada
Synopsis
Though white matter has a noted role in motor learning, there have been no MRI studies of functional neuroplasticity in this tissue. Therefore, in this work, twelve healthy participants underwent a motor training program designed to drive behavioral changes in the non-dominant hand. Using BOLD fMRI, we noted an associated change in the temporal dispersion of the white matter hemodynamic response over the training period. This is in line with previous DTI studies that show increases in white matter myelination with training, and BOLD investigations that show hemodynamic responses differ between grey and white matter, and between white matter tracts.
INTRODUCTION
White matter has a noted role in motor learning, with histological studies showing that when the formation of new myelin is blocked, mice cannot learn new skills (1). These investigations have been extended to human models as well, with numerous longitudinal diffusion tensor imaging studies showing that white matter tracts are modified by motor training (2,3,4). Despite this, there have been no studies investigating functional neuroplasticity in white matter. This is partially due to a lack of established MRI methods for investigating white matter function, however, the application of BOLD fMRI to white matter is becoming increasingly accepted. Though activation in this tissue is lower amplitude, high-field scanners are increasingly able to detect it, and modifications of analysis techniques are helping reduce the bias towards the detection of only gray matter activation (5). Crucially, it has been noted that the hemodynamic responses of white matter differ from gray matter (6), and even differ between white matter tracts (7). Noting this, our investigation set out to drive motor learning in our participants, and utilized BOLD fMRI to investigate the patterns of activation in white matter, as well as their amplitude and temporal changes associated with motor learning.METHODS
Twelve (12) healthy, right handed participants (male:female=5:7) completed a fine motor and a gross motor task with both hands during a series of three MRI scans, each separated by a week of training. Participants were randomized into either a fine motor or a gross motor training for the first week, which they completed on their personal computer before returning for their midpoint scan, and then switched into training for the other task.The training consisted of a fine motor “tracing” task, in which participants used a computer mouse to guide a cursor through a trace presented on a screen in front of them, attempting to maximize speed while minimizing errors. The gross motor task consisted of the same visual cues, but instead participants “coloured” in the background, reducing the need for fine motor control. The fine motor task was designed to be more difficult for the non-dominant hand, allowing for a comparison between the training effect with the dominant hand. This work focused on the dominant/non-dominant hand contrast.
Functional scans were completed using a Philips FFE single-shot GRE-EPI sequence on a 3T Ingenia CX scanner, and data were pre-processed using FSL. Analysis used a linear optimal basis set for the hemodynamic response function (HRF) convolution generated in FLOBS, to better reflect WM hemodynamics. Three bases were generated; an “HRF-like” curve, a “latency derivative” that modelled the temporal lag of the HRF, and a “dispersion derivative” that modelled differences in width of the HRF. The bases were convolved with the stimulus time series to determine group differences of both HRF amplitude and associated temporal characteristics (7).
RESULTS
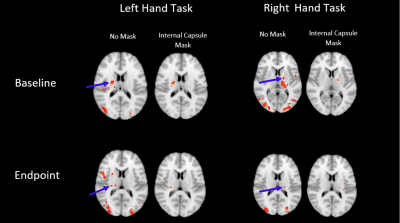
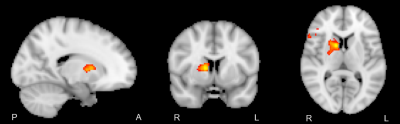
A two-way repeated measures ANOVA and post-hoc t-tests confirmed that the training effect between baseline and endpoint was confined to the left-hand fine motor task (p<0.005). [XS1] In order to pursue this non-dominant/dominant hand comparison, a baseline vs endpoint comparison revealed significant local maxima of activation within the internal capsule (z>2.5 P = 0.05) in a lateralized manner, depending on the hand used in the task (Figure 1). Further investigation of activation changes between baseline and endpoint revealed no differences in the amplitude or extent of the BOLD signal, but a significant change in HRF dispersion was noted (z >2.5, P = 0.05, FWE corrected), only in the left-hand fine motor condition, consistent with our behavioural findings (Figure 2).DISCUSSION
In line with previous findings, BOLD activation was detectable in the internal capsule in a lateralized manner. Though activation could be expected to be present throughout the corticospinal tract, this and previous reports only were able to detect activation in the internal capsule. This may be the result of anisotropic orientation of vasculature in the corticospinal tract selectively dampening BOLD signal due to parallel orientation to the main magnetic field of the scanner (8). Significant changes in the dispersion derivative of this activation were also noted when comparing endpoint relative to baseline, representing a decrease in the width of the HRF over the course of the training period. This was consistent with the noted variance of white matter hemodynamic responses between different areas, showing that changes over time are also possible. Overall, the functional MRI results were consistent with prior diffusion tensor imaging studies of neuroplasticity in white matter, which show structural changes in white matter myelination.CONCLUSION
Though there have been a number of studies investigation changes in white matter structure with motor training, this is to our knowledge the first report of MRI detectable functional neuroplasticity in white matter. It represents an important step forward into a new field, as it shows functional changes in white matter are present and helps guide future work by highlighting the importance of including measures of temporal characteristics when investigating hemodynamic responses within white matter.Acknowledgements
No acknowledgement found.References
1. McKenzie, I. A., Ohayon, D., Li, H., De Faria, J. P., Emery, B., Tohyama, K., & Richardson, W. D. (2014). Motor skill learning requires active central myelination. Science, 346(6207), 318–322.
2. Reid, L. B., Sale, M. V., Cunnington, R., Mattingley, J. B., & Rose, S. E. (2017). Brain changes following four weeks of unimanual motor training: Evidence from fMRI-guided diffusion MRI tractography. Human Brain Mapping, 38(9), 4302–4312. https://doi.org/10.1002/hbm.23514
3. Scholz, J., Klein, M. C., Behrens, T. E. J., & Johansen-Berg, H. (2009). Training induces changes in white-matter architecture. Nature Neuroscience, 12(11), 1370.
4. Taubert, M., Draganski, B., Anwander, A., Muller, K., Horstmann, A., Villringer, A., & Ragert, P. (2010). Dynamic Properties of Human Brain Structure: Learning-Related Changes in Cortical Areas and Associated Fiber Connections. Journal of Neuroscience, 30(35), 11670–11677. https://doi.org/10.1523/JNEUROSCI.2567-10.2010
5. Grajauskas, L. A., Frizzell, T., Song, X., & D’Arcy, R. C. N. (2019). White Matter fMRI Activation Cannot Be Treated as a Nuisance Regressor: Overcoming a Historical Blind Spot. Frontiers in Neuroscience, 13. https://doi.org/10.3389/fnins.2019.01024
6. Courtemanche, M. J., Sparrey, C. J., Song, X., MacKay, A., & D’Arcy, R. C. N. (2018). Detecting white matter activity using conventional 3 Tesla fMRI: An evaluation of standard field strength and hemodynamic response function. NeuroImage, 169, 145–150. https://doi.org/10.1016/j.neuroimage.2017.12.008
7. Ward, N. S., Swayne, O. B. C., & Newton, J. M. (2008). Age-dependent changes in the neural correlates of force modulation: An fMRI study. Neurobiology of Aging, 29(9), 1434–1446.
8. Hernández-Torres, E., Kassner, N., Forkert, N. D., Wei, L., Wiggermann, V., Daemen, M., … Rauscher, A. (2017). Anisotropic cerebral vascular architecture causes orientation dependency in cerebral blood flow and volume measured with dynamic susceptibility contrast magnetic resonance imaging. Journal of Cerebral Blood Flow & Metabolism, 37(3), 1108–1119. https://doi.org/10.1177/0271678X16653134
Figures