1791
Importance of Off-resonance Effects in Ultrashort echo-time Imaging1Center for Neuroscience and Regenerative Medicine, NIH/USU, Bethesda, MD, United States, 2Radiology and Imaging Sciences, NIH, Bethesda, MD, United States
Synopsis
Ultrashort echo-time (UTE) imaging can detect short- and ultrashort-T2 tissue components. e.g. tendons, ligaments, and cortical bone. Multi-echo UTE is used to generate the tissue attenuation maps required for quantitative MRI PET, as short T2 skull is visible on the first echo but not on the second. For accurate classification, it is assumed that these are registered. Here we show that geometric scaling issues of fat and water may be different between the 1st and 2nd echoes – and hence lead to erroneous tissue classification.
INTRODUCTION
Multi echo UTE has been used for identification of short T2 tissues based on the large drop in signal between the echoes1, 3, particularly the first and second echoes, to identify ultrashort T2 tissues, which are otherwise indistinguishable from air. For signal change between the two echoes to be valid, there is an implicit assumption that the data from the two echoes at each voxel correspond to the same tissue, i.e. the first and second echoes are anatomically coregistered. Here we show, for particular implementations of a UTE pulse sequence with a kooshball readout, that (1) the first echo is overall scaled smaller than the second and (2) that within the first echo, chemical shift results in an overlap between fat and water that is not present in the second echo.METHODS
MRI acquisition:MR images were acquired using the vendor provided two-echo 3D UTE pulse sequence with a kooshball readout (ute3d2_ns) on a Siemens Biograph mMR 3T MRI system (Siemens, Erlangen, Germany) at software VE11P. Contrast parameters were TR = 11.94 ms, TE1 = 0.07 ms, TE2 = 2.46 ms, and flip angle = 10°. Geometric parameters were: image matrix 192×192×192, and voxel size of 1.56×1.56×1.56 mm.
Phantom Experiments
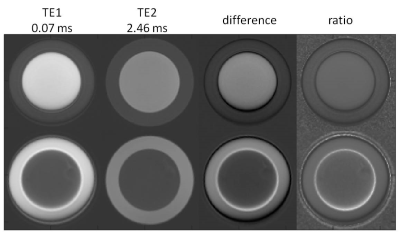
Two concentric oil/water phantoms were used, with an approximately 10 cm diameter inner container fitted concentrically within a 15 cm diameter container. For phantom 1, the “W-o” phantom, vegetable oil filled the inner container and water in the outer ring. For phantom 2, the “O-w” phantom, the contents were reversed.
Healthy volunteer experiments:
HV volunteers were scanned under an IRB approved protocol (NCT00001711) using the above MRI acquisition. UTE images of the head were obtained.
RESULTS
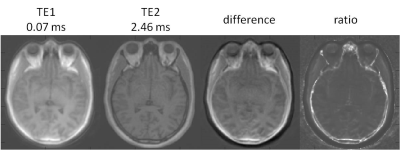
In Figure 1, first- and second-echo images of kooshball UTE acquisitions from the two phantoms were compared. Dependent on whether the oil was at the center or not, there were a gap (W-o phantom) or an overlap (O-w phantom) between the water and oil. This was clearly seen in the difference and ratio images. Furthermore, the diameter of the phantoms on TE1 was smaller than on TE2. Similar off-resonance effects were seen in brain images, as shown in Figure 2. The head size on TE1 was smaller than on TE2. On TE1, skull bone marrow fat overlapped with meninges, which is between the skull and brain.DISCUSSION
Because both echoes of the UTE are obtained synchronously, true anatomical coregistration between the two sets of data is expected. However, it may be the case that the gradients played out during acquisition are not necessarily identical, perhaps due to sequence design or hardware limitations. Furthermore, chemical shift between fat and water can result in differential overlap of fat and water between the two echoes. Both processes result in artifactual signal changes between the 1st and 2nd echo which can be misattributed to the presence of e.g. bone. We showed the degradation of UTE image quality due to off-resonance fat signals. It manifests as artifactual rings, erroneous overlap with water signal, and incorrect overall scaling of the first-echo images. It is essential to have proper corrections, such as fat-water separation2, 5, 8, careful gradient calibrations4, 7, and/or improved image reconstruction, so that multi-echo UTE imaging can give accurate quantitation of altra-short T2 components. Without corrections, incorporating the altrashort TE image with late TEs and other images may workaround the issue of fat-water chemical shift. For example, improved µmaps can be calculated based non-supervised training using two-echo UTE images and CT images6.CONCLUSION
It is essential to correct off-resonance effects for multi-echo UTE imaging to become a commonly useful imaging modality.Acknowledgements
This study was supported by the Department of Defense in the Center for Neuroscience and Regenerative Medicine and the Intramural Research Program of the National Institutes of Health.References
1 T. Boucneau, P. Cao, S. Tang, M. Han, D. Xu, R. G. Henry, and P. E. Z. Larson, 'In Vivo Characterization of Brain Ultrashort-T2 Components', Magn Reson Med, 80 (2018), 726-35.
2 E. K. Brodsky, J. H. Holmes, H. Yu, and S. B. Reeder, 'Generalized K-Space Decomposition with Chemical Shift Correction for Non-Cartesian Water-Fat Imaging', Magn Reson Med, 59 (2008), 1151-64.
3 J. Du, G. Ma, S. Li, M. Carl, N. M. Szeverenyi, S. VandenBerg, J. Corey-Bloom, and G. M. Bydder, 'Ultrashort Echo Time (Ute) Magnetic Resonance Imaging of the Short T2 Components in White Matter of the Brain Using a Clinical 3t Scanner', Neuroimage, 87 (2014), 32-41.
4 K. H. Herrmann, M. Kramer, and J. R. Reichenbach, 'Time Efficient 3d Radial Ute Sampling with Fully Automatic Delay Compensation on a Clinical 3t Mr Scanner', PLoS One, 11 (2016).
5 S. B. Reeder, Z. Wen, H. Yu, A. R. Pineda, G. E. Gold, M. Markl, and N. J. Pelc, 'Multicoil Dixon Chemical Species Separation with an Iterative Least-Squares Estimation Method', Magn Reson Med, 51 (2004), 35-45.
6 S. Roy, W. T. Wang, A. Carass, J. L. Prince, J. A. Butman, and D. L. Pham, 'Pet Attenuation Correction Using Synthetic Ct from Ultrashort Echo-Time Mr Imaging', J Nucl Med, 55 (2014), 2071-7.
7 H. Tan, and C. H. Meyer, 'Estimation of K-Space Trajectories in Spiral Mri', Magn Reson Med, 61 (2009), 1396-404.
8 D. Wang, N. R. Zwart, and J. G. Pipe, 'Joint Water-Fat Separation and Deblurring for Spiral Imaging', Magn Reson Med, 79 (2018), 3218-28.
Figures