1785
Metabolite activity in the anterior cingulate cortex during a painful stimulus using functional MRS1Experimental Medicine, University of British Columbia, Vancouver, BC, Canada, 2International Collaboration on Repair Discoveries (ICORD), Vancouver, BC, Canada, 3Radiology, University of British Columbia, Vancouver, BC, Canada, 4Image Tech Lab, Simon Fraser University, Vancouver, BC, Canada, 5Philips Healthcare Canada, Vancouver, BC, Canada, 6Physics and Astronomy, University of British Columbia, Vancouver, BC, Canada, 7Radiology, University of British Columbia, vancouver, BC, Canada, 8UBC MRI Research Centre, Vancouver, BC, Canada, 9Physics and Astronomy, University of British Columbia, vancouver, BC, Canada, 10Pathology and Laboratory Medicine, University of British Columbia, Vancouver, BC, Canada, 11School of Kinesiology, University of British Columbia, Vancouver, BC, Canada
Synopsis
Current treatment evaluation procedures of pain conditions are dependent on self-reported measures. The objective of this study was to determine changes in excitatory neurotransmitters (i.e., glutamate and glutamate+glutamine) in the anterior cingulate cortex as an objective measure of pain during a painful stimulus using single voxel functional magnetic resonance spectroscopy (fMRS). Glutamate concentration changes during the painful stimulus suggest a role for glutamate in detecting pain which was not related to self-reported pain ratings. An exploratory analysis on sex revealed an 8.63% (p=0.08) increase in glutamate at pain onset in female participants compared with a 7.45% (p=0.31) increase in males.
Introduction
Magnetic resonance spectroscopy (MRS) can quantify brain metabolites including n-acetyl-aspartate (NAA), total creatine (tCr), glutamate (Glu), and glutamate+glutamine (Glx). Functional MRS (fMRS) monitors metabolites over time, is sensitive to task-related changes in neurotransmitters1–6 and can elucidate neural mechanisms underlying information processing (i.e., sensory stimulation)1,4,6–10. fMRS studies examining changes in metabolites in relation to painful stimulus in healthy subjects, have primarily focused on the anterior cingulate cortex (ACC)1,2,7,11,12, an area active during pain stimulation and pain-relief interventions13–15. Our goal was to examine the relationship between ACC metabolite activity and pain perception in healthy controls using fMRS. We hypothesize that increased Glu concentrations in the ACC (1) would occur in relation to pain, and (2) are associated with pain ratings.Methods
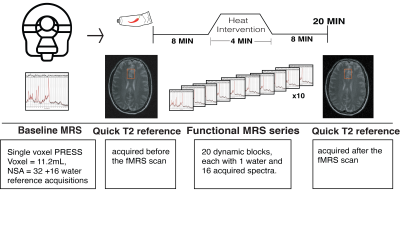
MR Experiments: Eighteen healthy participants (9F/9M, mean age=26.2 SD=3.68, range=21-36yrs) were recruited. 3T data (Philips Achieva, transmit-receive head coil) included:(1) 3DT1 (MPRAGE, TE/TR/TI=3.5/7.7/808ms, shot interval=1800ms, 1mm³ isotropic resolution, FOV (ap/rl/fh)=256/200/150mm³)
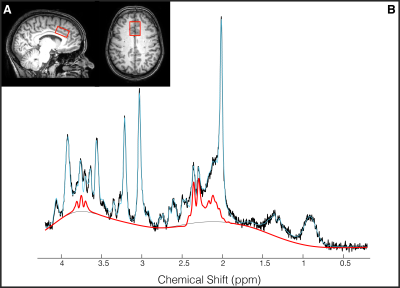
(2) 1H-MRS (PRESS, TE/TR=22/4000ms, NSA=32, ACC voxel size=30/25/15mm3 =11.2mL, 2nd order shimming, 16-step phase cycle with water suppression using the Excitation option– a Philips variant of CHESS16; a non-water suppressed acquisition preceded each complete phase-cycle for absolute metabolite quantification4 and water signal monitoring, Figure 1A)
(3) T2-weighted (TE/TR=90/2000ms, FOV (ap/rl/fh) = 250/189/36mm³, resolution=1x1x3 mm3).
Pain intervention: Capsaicin (0.075% topical) was applied on the right forearm volar surface. After 8 minutes of “pre-heat” fMRS, a thermo-pad was activated for 4.4 minutes by circulating ~41°C heated water (“heat”). fMRS was acquired continuously during heat application and for 8 minutes after heat (“post heat”) (Figure 2). During fMRS participants provided pain ratings every 2-minutes using the 0-10 numeric rating scale (NRS)17 via an MRI-compatible clicker .
Data Analysis: 3DT1 data was segmented into white matter, gray matter and cerebrospinal fluid using FSL BET and FAST18. Individual FIDs were pre-processed (eddy current correction, frequency alignment, visual inspection) in MATLAB (R2016b). 32 shots were averaged for each 2-minute block, yielding a total of 11 spectra analyzed using LCModel (v6.3-1H) (Figure 1B). The corresponding interleaved non-water suppressed spectra and each participant’s brain water tissue volume were used to calculate the concentration of NAA, tCr, glutamate and Glx in millimolar (mM)19–22. Individual water FID’s were fitted to a single-exponential decay curve to extrapolate the water amplitude.
Statistical Analysis: A paired t-test determined changes in metabolites at the onset of self-reported pain. A linear mixed effects model determined the relationship between metabolites (Glu, Glx) and pain ratings, including random slopes and intercepts. Sex-effect was explored using Cohen’s d23 = group mean difference/pooled standard deviation. Effect size quantifies the difference between two groups where the size of the difference is emphasized rather than confounding this with sample size (small effect: d=0.2, medium effect: d=0.5, large effect: d=0.8). A Pearson correlation assessed the relationship between water amplitude and metabolite concentrations. All statistics were performed using R (v1.1.442).
Results
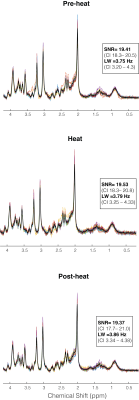
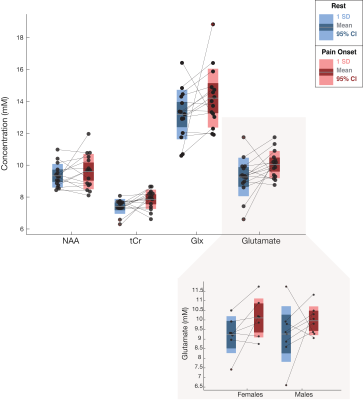
One participant (F) was unable to be scanned and 2 participants’ (1M/1F) data sets were rejected due to motion. The 15 remaining subjects had very consistent high signal to noise ratio and narrow line widths (Figure 3). Average reported pain ratings were 3.8±2.0 during heat and 4.2±1.9 post-heat.Trends of increased Glu (+8.38%, p=0.06, t=2.04), Glx (+7.81%, p=0.11, t=1.74) and tCr (+6.0%, p=0.05, t=2.06) were observed at pain onset relative to rest, with medium-large effects (Cohen’s dGlu=0.74 CI=-0.05–1.55; Cohen’s dGlx=0.60 CI=-0.18–1.40; Cohen’s dtCr= 0.83 CI= 0.02-1.64). NAA showed no change at pain onset compared to rest (2.81%, p=0.34, t=0.9, small effect Cohen’s dNAA=0.28 CI=-0.49–1.05) (Figure 4).
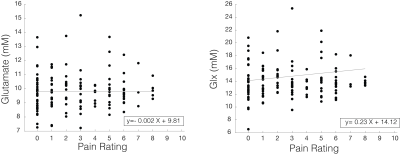
Glu and Glx were not significantly correlated with pain ratings across the fMRS acquisition (Glu: ß±standard error=0.017±0.06, p=0.79; Glx: -0.004±0.13, p=0.97) (Figure 5).
Exploratory sex-based analysis revealed large changes in Glu at pain onset in females (8.63%, p=0.08, t=2.1,Cohen’s dfemale=0.88, CI=-0.43–2.21), compared to medium effects in males (7.45%, p=0.31, t=1.0, Cohen’s dmale=0.60 CI=-0.57–1.73), although pain ratings were not different between the groups (Figure 4). There was no relationship between any metabolite (Glu, Glx, NAA, tCr) and water amplitude (r=0.11, p=0.63;r=-0.24 p=0.31; r=0.008, p=0.97; r=0.26, p=0.27, respectively).
Discussion
The relationship between ACC metabolite concentrations and noxious heat-derived pain was examined using fMRS. At pain onset, trend-level increases with medium to large effect sizes were detected for Glu, Glx and tCr, while NAA remained constant. The trend for Glu to increase during the first perception of pain was higher in females, which may be related to the effect of steroidal sex hormones on glutamate-glutamine metabolism in the brain24.Increased tCr with pain perception may indicate its role in neuromodulation25–27, since creatine and its precursor can act as neuromodulators of GABAergic neurons25. Neither Glu nor Glx levels were related to pain ratings over time, which may be due to the transient nature of this increase and/or the subjective nature of self-reported pain levels. The lack of association between metabolite concentration and water amplitude suggests changes in water did not drive changes in these concentrations.
Conclusion
Functional MRS in the anterior cingulate cortex demonstrated Glu, Glx and tCr concentration increases during a pain stimulus which were not related to self-reported pain ratings. Preliminary evidence suggests males and females experience pain differently, which may be important to consider when developing novel avenues for sex-specific treatments.Acknowledgements
We would like to thank the University of British Columbia MRI Research Centre, the International Collaboration for Repair Discoveries (ICORD) as well as volunteers who participated in the study.
J. Archibald is supported by a research scholarship of the National Council of Science and Technology (CONACYT), GSM-NSERC and the Michel Smith foreign study supplement (MSFSS). E.L MacMillan receives salary support from Philips Canada. CG is supported by an endMS Master’s studentship award from the MS Society of Canada. CL holds operating grant funding from the MS Society of Canada and the Natural Sciences and Engineering Research Council of Canada (NSERC). This work is supported by NSERC Program Discovery grant held by JLKK.
References
1. Gussew, A. et al. Time-resolved functional 1H MR spectroscopic detection of glutamate concentration changes in the brain during acute heat pain stimulation. Neuroimage 49, 1895–1902 (2010).
2. Cleve, M., Gussew, A. & Reichenbach, J. R. In vivo detection of acute pain-induced changes of GABA+ and Glx in the human brain by using functional 1H MEGA-PRESS MR spectroscopy. Neuroimage 105, (2014).
3. Chiappelli, J. et al. Glutamatergic Response to Heat Pain Stress in Schizophrenia. Schizophr. Bull. 1–10 (2017). doi:10.1093/schbul/sbx133
4. Bednařík, P. et al. Neurochemical and BOLD responses during neuronal activation measured in the human visual cortex at 7 Tesla. J. Cereb. Blood Flow Metab. 35, 601–610 (2015).
5. Apšvalka, D., Gadie, A., Clemence, M. & Mullins, P. G. Event-related dynamics of glutamate and BOLD effects measured using functional magnetic resonance spectroscopy (fMRS) at 3T in a repetition suppression paradigm. Neuroimage 118, 292–300 (2015).
6. Mullins, P. G. Towards a theory of functional magnetic resonance spectroscopy (fMRS): A meta-analysis and discussion of using MRS to measure changes in neurotransmitters in real time. Scand. J. Psychol. 59, 91–103 (2018).
7. Mullins, P. G., Rowland, L. M., Jung, R. E. & Sibbitt, W. L. A novel technique to study the brain’s response to pain: Proton magnetic resonance spectroscopy. Neuroimage 26, 642–646 (2005).
8. Stanley, J. A. & Raz, N. Functional magnetic resonance spectroscopy: The ‘new’ MRS for cognitive neuroscience and psychiatry research. Front. Psychiatry 9, (2018).
9. Taylor, R. et al. Functional magnetic resonance spectroscopy of glutamate in schizophrenia and major depressive disorder: anterior cingulate activity during a color-word Stroop task. npj Schizophr. 1, 15028 (2015).
10. Cleve, M., Gussew, A., Wagner, G., Bär, K. J. & Reichenbach, J. R. Assessment of intra- and inter-regional interrelations between GABA+, Glx and BOLD during pain perception in the human brain – A combined1H fMRS and fMRI study. Neuroscience 365, 125–136 (2017).
11. Kupers, R., Danielsen, E. R., Kehlet, H., Christensen, R. & Thomsen, C. Painful tonic heat stimulation induces GABA accumulation in the prefrontal cortex in man. Pain 142, 89–93 (2009).
12. Zunhammer, M. et al. Combined glutamate and glutamine levels in pain-processing brain regions are associated with individual pain sensitivity. Pain 157, 1 (2016).
13. Casey, K. L. et al. Selective opiate modulation of nociceptive processing in the human brain. J. Neurophysiol. 84, 525–533 (2000).
14. Petrovic, P., Kalso, E., Petersson, K. M. & Ingvar, M. Placebo and opioid analgesia - Imaging a shared neuronal network. Science (80-. ). 295, 1737–1740 (2002).
15. deCharms, R. C. et al. Control over brain activation and pain learned by using real-time functional MRI. Proc. Natl. Acad. Sci. U. S. A. 102, 18626–31 (2005).
16. Haase, A., Frahm, J., Hanicke, W. & Matthaei, D. 1H NMR chemical shift selective (CHESS) imaging. Plasma Sources Sci. Technol. (1985). doi:10.1088/0031-9155/30/4/008
17. Hawker, G. A., Mian, S., Kendzerska, T. & French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 63, 240–252 (2011).
18. Zhang, Y; Brady, M; Smith, S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging 20, 45–57 (2001).
19. MacMillan, E. L. et al. Progressive multiple sclerosis exhibits decreasing glutamate and glutamine over two years. Mult. Scler. J. 22, 112–116 (2016).
20. Liang, A. L. W. et al. Short-term stability of T 1 and T 2 relaxation measures in multiple sclerosis normal appearing white matter. J. Neurol. 259, 1151–1158 (2012).
21. Laule, C. et al. Long T2 water in multiple sclerosis: What else can we learn from multi-echo T2 relaxation? J. Neurol. 254, 1579–1587 (2007).
22. de Graaf, R. in vivo NMR Spectroscopy Principles and Techniques. (2007). doi:10.1080/09297040802385400
23. Cohen, J. Statistical Power Analysis for the Behavioral Sciences. Routledge. ISBN 978-1-134-, (1988).
24. Haghighat, N. Estrogen (17β-Estradiol) enhances glutamine synthetase activity in C6-glioma cells. Neurochem. Res. 30, 661–667 (2005).
25. Koga, Y. et al. Brain creatine functions to attenuate acute stress responses through GABAnergic system in chicks. Neuroscience 132, 65–71 (2005).
26. Van De Kamp, J. M., Jakobs, C., Gibson, K. M. & Salomons, G. S. New insights into creatine transporter deficiency: The importance of recycling creatine in the brain. J. Inherit. Metab. Dis. 36, 155–156 (2013).
27. Almeida, L., Salomons, G., Hogenboom, F., Jakobs, C. & Schoffelmeer, A. Exocytotic Release of Creatine in Rat Brain. Synapse 346, 319–346 (2006).
Figures