1784
Development of an unbiased population-specific brain atlas for adolescent collision-sport athletes1Weldon School of Biomedical Engineering, Purdue University, West Lafayette, IN, United States, 2College of Veterinary Medicine, Purdue University, West Lafayette, IN, United States, 3Department of Statistics, Purdue University, West Lafayette, IN, United States, 4Department of Basic Medical Sciences, Purdue University, West Lafayette, IN, United States, 5School of Electrical and Computer Engineering, Purdue University, West Lafayette, IN, United States, 6Department of Health and Kinesiology, Purdue University, West Lafayette, IN, United States, 7School of Health Sciences, Purdue University, West Lafayette, IN, United States, 8School of Mechanical Engineering, Purdue University, West Lafayette, IN, United States
Synopsis
Over years of practices and competitions, adolescent collision-sport (American football, soccer) athletes undergo repetitive subconcussive head impacts, and therefore may exhibit a neuroanatomical trajectory different from healthy adolescents in general. Targeting this vulnerable population, we constructed a specific brain atlas that includes templates (T1 and DTI) and semantic labels (cortical and white matter parcellations), and we demonstrated that the unbiased population-specific brain atlas can minimize bias introduced in spatial normalization, improve sensitivity of voxel-wise statistical analysis, and therefore better clarify the mechanisms that lead to traumatic brain injury in adolescent athletes.
Introduction
Concussion and subconcussive trauma adversely impact the brain health of adolescents who participate in collision sports1,2. Nevertheless, current DTI literature of sport-related mild traumatic brain injury (mTBI) reported inconsistent changes of metrics (e.g. fractional anisotropy) in variable white matter regions, largely differed in analytic techniques, experimental designs, and scanning parameters3, obscuring an unequivocal voice being made for this vulnerable population. In group analysis, individual brain images are typically normalized to a common space, using a stereotaxic brain atlas. However, when the underlying neuroanatomy of the chosen atlas does not represent the study population, greater biases and errors may be introduced, confounding subsequent statistical findings. We hypothesize that adolescent collision-sport (American football, soccer) athletes exhibit a neuroanatomical trajectory different from healthy adolescents in general, and therefore demands an unbiased population-specific brain atlas for group analysis. Here, utilizing a previously established workflow4, we created templates (T1 and DTI) and semantic labels (cortical and white matter parcellations), based on the longitudinal database at Purdue Neurotrauma Group (PNG)5. The atlas has been made available at https://doi.org/10.4231/XGNK-JX08 for downloading.Methods
All the data used for atlas construction were collected in PNG’s ongoing longitudinal study of adolescent athletes5 (see Table 1), using a 3 Tesla GE Signa HDx (Waukesha, WI) with a 16-channel brain array (Nova Medical; Wilmington, MA). Data were acquired approximately one month before contact practices began (Pre), within competition season (In), and after the season ended (Post). T1 scans were acquired using a 3D FSPGR sequence (TR/TE=5.7/2.0ms, flip angle=73°, 1mm isotropic resolution); DWI data were acquired using a spin-echo EPI sequence (TR/TE=12,500/100ms, 40 slices with 2.5mm thickness, matrix=96×96, upsampled to 1mm isotropic resolution), with 30 diffusion-encoding directions at b=1000s/mm2 and one at b=0s/mm2. Preprocessing of T1 data included denoising, bias correction, skull-stripping, and intensity normalization; preprocessing of DWI data included motion and eddy current corrections, followed by brain extraction. Fractional anisotropy (FA) was estimated for each individual, and all the data underwent visual quality inspection. The population-specific T1 and DTI templates were created using ANTs6, and the semantic labels were created using the recon-all pipeline of Freesurfer7. To evaluate the T1 templates, T1 scans of 12 male football athletes acquired in season 2018-2019 were nonlinearly registered to ICBM152, an age-appropriate pediatric template (NIHPD13.5-18.5)8, IITv3.09, and PNG T1 template. The maps of absolute logarithm of Jacobian determinant (logJ, representing local volume difference) were computed and transformed to the common ICBM152 space, and analyzed using voxel-wise statistics with 5000 permutations. To evaluate the DTI templates, FA maps of 64 male football athletes scanned at Pre and the second 6-week halves of the season (In2) were first aligned to FMRIB58 (Oxford, UK), IITv3.09, and PNG DTI template. All aligned FA images were then normalized to the ICBM152 space. Tract-based spatial statistics (TBSS)10 were used to create FA skeletons, followed by voxel-wise statistics (5000 permutations). The FA skeletons were segmented into 48 ROIs using the JHU-ICBM-DTI-81 atlas11. Non-parametric Friedman test was performed to test whether the total number of voxels within the ROI (Vtotal) correlates with the templates. Logistic regression was performed to test whether the ratio of the number of significant voxels (Vs, from the voxel-wise statistics) and Vtotal correlates with the selected templates.Results
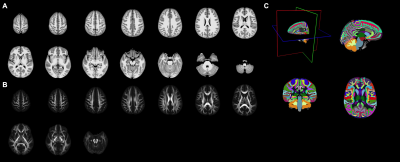
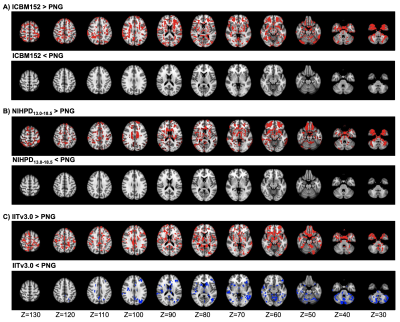
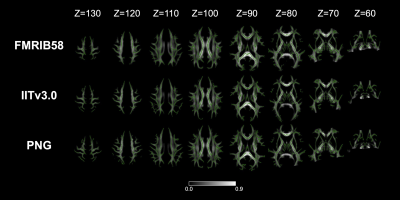
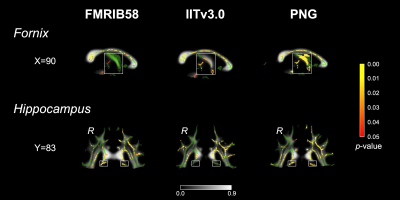
The population-specific brain atlas contains T1 (Figure 2A) and DTI (Figure 2B) templates, and semantic labels (Figure 2C). Compared to ICBM152 (Figure 3A) or NIHPD13.0-18.5 (Figure 3B), no significantly larger logJ was produced from using PNG T1 template for the spatial normalization. Compared to IITv3.0 (Figure 3C), fewer voxels showed significantly larger logJ when using PNG template (IITv3.0: 334811; PNG: 109189). The TBSS skeletons were similar across the templates (Figure 4), and template was not a significant covariate for Vtotal (χ2=2.370, p=0.499) but was significant for Vs/Vtotal (χ2=9.759, p=0.020). For PNG DTI template, the significant voxels in fornix were 99mm3, much larger compared to FMRIB58 (14mm3) and IITv3.0 (5mm3); for IITv3.0 DTI template, no significant voxel of FA difference was observed in bilateral hippocampi (Figure 5).Discussion
Using PNG T1 template introduced minimal bias during spatial normalization of the T1 images from local adolescent collision-sport athletes (Figure 3). Template selection did not lead to significantly different TBSS skeleton (Figure 4), but was a significant covariate for the voxel-wise statistical analyses. Compared to FMRIB58, the PNG template resulted in consistent but more sensitive detections of FA decrease within the fornix and bilateral hippocampi, whereas on the skeleton of IITv3.0, such difference was either detected with fewer voxels or not significant (Figure 5). In the future, the PNG templates may serve as an coordinate reference system to retrospectively harmonize the DWI data collected from different sites and acquisition parameters12, and the semantic labels may be applied to investigate volumetric trajectory of collision-sport athletes during adolescence13.Conclusion
Compared to the standardized brain atlases, the population-specific brain atlas better characterized the neuroanatomy of the adolescent collision-sport athletes, reduced biases introduced during spatial normalization, and exhibited higher sensitivity in detecting regional FA differences. In summary, we demonstrated that template selection is a critical strategic step towards reproducible and meaningful statistical results, and the unbiased population-specific brain atlas can better clarify the mechanisms of traumatic brain injury in adolescent athletes.Acknowledgements
This work was funded in part by grants from the Purdue Research Foundation, the Indiana Clinical and Translational Sciences Institute Spinal Cord and Brain Injury Research Fund (SCBI #207-5 and #207-32), the BrainScope Company (as part of a grant from the GE-NFL Head Health Initiative), and the National Institutes of Health National Institute of Biomedical Imaging and Bioengineering (R03EB026231). This research was done using resources provided by the Open Science Grid, which is supported by National Science Foundation award 1148698 and the U.S. Department of Energy's Office of Science. This research was supported in part by community cluster provided by Information Technology at Purdue, West Lafayette, Indiana.
References
1. McCrea MA. Mild Traumatic Brain Injury and Postconcussion Syndrome: The New Evidence Base for Diagnosis and Treatment. New York: Oxford University Press; 2008. 205 p.
2. Nauman EA, Talavage TM. Subconcussive trauma. Handb Clin Neurol. 2018 Jan 1;158:245–55.
3. Asken BM, DeKosky ST, Clugston JR, Jaffee MS, Bauer RM. Diffusion tensor imaging (DTI) findings in adult civilian, military, and sport-related mild traumatic brain injury (mTBI): a systematic critical review. Brain Imaging Behav. 2018;12(2):585–612.
4. Zou Y, Zhu W, Yang H-C, Zhu Y, Tong Y, Talavage TM, et al. Develop Population-specific Brain Atlas with High-throughput High-performance Computingle. In: Proceedings of the 25th Annual Meeting of the Organization for Human Brain Mapping. Rome, Italy; 2019.
5. Talavage TM, Nauman EA, Leverenz LJ. The role of medical imaging in the recharacterization of mild traumatic brain injury using youth sports as a laboratory. Front Neurol. 2016;6:273.
6. Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12(1):26–41.
7. Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774–81.
8. Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 2011;54(1):313–27.
9. Zhang S, Arfanakis K. Evaluation of standardized and study-specific diffusion tensor imaging templates of the adult human brain: Template characteristics, spatial normalization accuracy, and detection of small inter-group FA differences. Neuroimage. 2018;172:40–50.
10. Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–505.
11. Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51(5):527–39.
12. Cetin Karayumak S, Bouix S, Ning L, James A, Crow T, Shenton M, et al. Retrospective harmonization of multi-site diffusion MRI data acquired with different acquisition parameters. Neuroimage. 2019;184:180–200.
13. Narvacan K, Treit S, Camicioli R, Martin W, Beaulieu C. Evolution of deep gray matter volume across the human lifespan. Hum Brain Mapp. 2017;38(8):3771–90.
Figures