1775
Feasibility of a quasi-volumetric synthetic MRI of the brain using 2D slice-interleaved MDME acquisition with deep learned reconstruction1Department of Radiology, Haeundae Paik Hospital, Busan, Republic of Korea, 2MR Collaboration and Development, GE Healthcare, Calagary, AB, Canada, 3GE Healthcare Korea, Seoul, Republic of Korea
Synopsis
Sequences that allow volumetric parametric maps are being developed but when these techniques will be available clinically is uncertain. MDME sequence is a robust method, allowing rapid acquisition of T1, T2 relaxation times, PD from which one can generate synthetic multi-contrast images. However, acquisition at thin slices is challenging. With a deep learned reconstruction method, we demonstrate that interleaved thin slice acquisition of MDME, can produce quasi-volumetric synthetic MRI at an isotropic resolution. Limitations include motion misregistrations between acquisitions, parallel imaging/partial volume/pulsation related artifacts, which we believe can be overcome with technical development.
Introduction
With the accumulation of evidence regarding quantitative MR imaging biomarkers, and increasing use of volumetric acquisitions for brain imaging, sequences that can yield volumetric multi-parametric maps are being introduced1-2 and validated3-4. However, these techniques are currently based on research use and availability is limited.A 2D multi-dynamic multi-echo (MDME)5 sequence for rapid simultaneous measurement of T1 and T2 relaxation times and proton density (PD), with correction of B1 field inhomogeneity, was previously proposed and was shown to be overall robust even across different scanners6. In addition, brain tissue volumes, and myelin volume fraction can be calculated. However, thin slice acquisition below 2 mm has not been widely adopted, presumably due to low SNR. Also, due to the cross talks, an inter-slice gap is recommended for MDME acquisition, thus continuous acquisition is not achievable in a single sequence.
DLRecon is a new deep learning-based MR reconstruction, which comprises a deep convolutional residual encoder network trained using a database of over 10,000 images to achieve images with high SNR and high spatial resolution, and can enable thin slice MDME acquisitions.
In this preliminary evaluation, we demonstrate a quasi-volumetric synthetic MRI method based on deep learned reconstruction of slice-interleaved MDME acquisition.
Methods
[Data Acquisition]Images were acquired on a 3T MRI scanner (Signa Architect, GE Healthcare, Waukesha, WI, USA), equipped with a 48-channel head coil.
The MDME sequence was acquired in the axial plane, with the following parameters: TR 6261 msec, TE 16.2/81.1 msec, FOV 208 × 177 mm, matrix size 160 x 160, slice thickness 1.3 mm with 1.3 mm interslice gap, ASSET factor 3, saturation delays calculated automatically). The acquisition was repeated with imaging slab shifted by 1.3 mm leading to an acquisition time of 4:36 x 2 (9:12). Each acquisition produced eight real and imaginary image pairs with different echo time and saturation delay combinations.
[Image Reconstruction]
The acquired data were retrospectively reconstructed with and without DLRecon at an empirical denoising level of 70% (DL70). T2, PD, T1FLAIR and PSIR contrasts were synthesized from the source images using software installed on the console (MAGIC, GE Healthcare, Waukesha, WI, USA).
[Post-processing]
With in-house-codes, interleaved images were combined and interslice variation of image intensity due to interleaved acquisition was corrected by a joint entropy-based weighted least squares estimation method7.
Results
While the images based on conventional reconstruction appear coarse, images synthesized from DL70 inputs show reduced noise (Figure 2.). When reformatted in the coronal or sagittal plane, there are ‘even-odd’ effects. These are reduced by the inter-slice intensity variation reduction method (Figure 3.). Reformats of the synthetic images can be achieved at an acceptable quality by the application of both DL70 and intensity standardization (Figure 4.).Discussion and Conclusion
There are still several limitations to this approach. First, registration between the interleaved stacks needs to be implemented to account for motion. Second, due to the high ASSET factor, there parallel imaging-related artifacts may be present at the mesial portion of the brain. Third, artifacts on FLAIR related to the partial volume are still present even at a thin slice (Figure 5.). Last but not least, the scan time, which takes approximately 9 minutes, is relatively long.These challenges may be overcome by further developments including implementation of motion correction8-9, methods to decrease artifacts10-12, and methods for further acceleration.
In conclusion, we have demonstrated a method utilizing deep learned reconstruction to acquire quasi-volumetric synthetic MRI of the brain, which have the potential for clinical application.
Acknowledgements
We thank Sang Hyuk Park for scanner arrangements.References
- Ma, D. et al. Development of high-resolution 3D MR fingerprinting for detection and characterization of epileptic lesions. J. Magn. Reson. Imaging 49, 1333–1346 (2019).
- Cheng, C. C., Preiswerk, F., Hoge, W. S., Kuo, T. H. & Madore, B. Multipathway multi-echo (MPME) imaging: all main MR parameters mapped based on a single 3D scan. Magn. Reson. Med. 81, 1699–1713 (2019).
- Fujita, S. et al. Three-dimensional high-resolution simultaneous quantitative mapping of the whole brain with 3D-QALAS: An accuracy and repeatability study. Magn. Reson. Imaging 63, 235–243 (2019).
- Fujita, S. et al. 3D quantitative synthetic MRI‐derived cortical thickness and subcortical brain volumes: Scan–rescan repeatability and comparison with conventional T 1 ‐weighted images. J. Magn. Reson. Imaging jmri.26744 (2019) doi:10.1002/jmri.26744.
- Milshteyn, E. et al. Development of high resolution 3D hyperpolarized carbon-13 MR molecular imaging techniques. Magn. Reson. Imaging 38, 152–162 (2017).
- Hagiwara, A. et al. Linearity, Bias, Intrascanner Repeatability, and Interscanner Reproducibility of Quantitative Multidynamic Multiecho Sequence for Rapid Simultaneous Relaxometry at 3 T. Invest. Radiol. 54, 39–47 (2019).
- Schmidt, M. A Method for Standardizing MR Intensities between Slices and Volumes. (2005) doi:10.7939/R3R49GG8D.
- Rohlfing, T., Rademacher, M. H. & Pfefferbaum, A. Volume reconstruction by inverse interpolation: Application to interleaved MR motion correction. in Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics) vol. 5241 LNCS 798–806 (2008).
- Nam, H., Lee, Y. J., Jeong, B., Park, H. J. & Yoon, J. Motion correction of magnetic resonance imaging data by using adaptive moving least squares method. Magn. Reson. Imaging 33, 659–670 (2015).
- Hagiwara, A. et al. Improving the quality of synthetic FLAIR images with deep learning using a conditional generative adversarial network for pixel-by-pixel image translation. Am. J. Neuroradiol. 40, 224–230 (2019).
- Ryu, K. et al. Data‐driven synthetic
MRI FLAIR artifact correction via deep neural network. J. Magn. Reson.
Imaging 50, 1413–1423 (2019).
- Eo, T. et al. KIKI-net: cross-domain convolutional neural networks for reconstructing undersampled magnetic resonance images. Magn. Reson. Med. 80, 2188–2201 (2018).
Figures
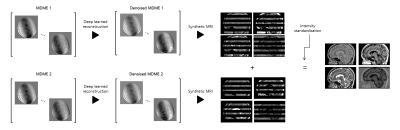
Figure 1. Summary of steps required to generate a quasi-volumetric synthetic MRI.
2 sets of 2D slice-interleaved MDME acquisitions are performed. Each set is denoised with deep learned reconstruction. Synthetic multi-contrast images are generated on scanner installed software. The images are then combined and intensity standardized using in-house developed code to produce quasi-volumetric images.
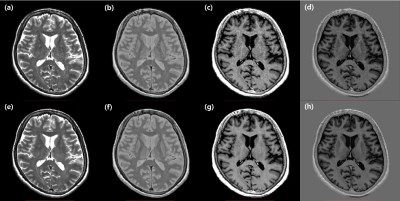
Figure 2. Representative slices of synthetic multi-contrast images.
Axial images synthesized from MDME acquisitions with (lower row) or without (upper row) deep learned reconstruction in T2 (a, e), PD (b, f), T1 FLAIR (c, g), and PSIR (d, h) contrasts are shown.
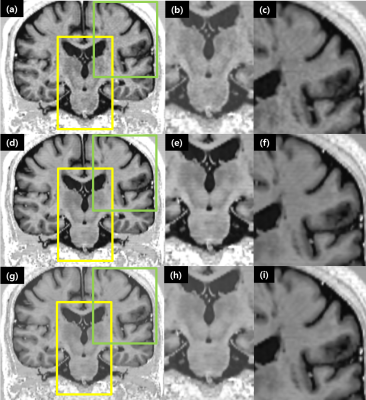
Figure 3. Representative slices of PSIR images showing the effect of inter-slice intensity variation reduction.Images without deep learned reconstruction.
(a, b, c), with deep learned reconstruction (d, e, f), with deep learned reconstruction and intensity normalization. The second and third columns are magnified views of the yellow and green boxes in the first column, respectively. The reduction of ‘even-odd’ offset effects is demonstrated.
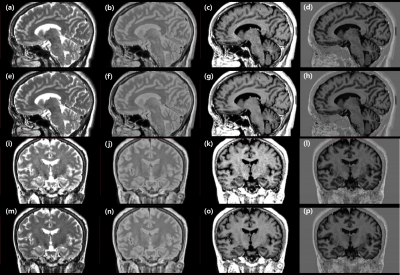
Figure 4. Representative slices of multi-contrast images reformatted to sagittal and coronal planes.
Images with (lower row) or without (upper row) deep learned reconstruction and intensity normalization, in T2 (a, e, i, m), PD (b, f, j, n), T1 FLAIR (c, g, k, o), and PSIR (d, h, l, p) contrasts are shown.
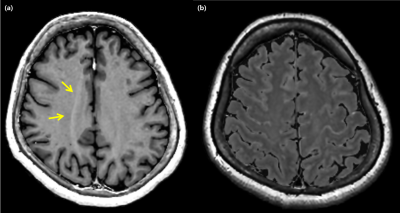
Figure 4. Image artifacts associated with the demonstrated method.
(a) ASSET related artifacts (arrows) are present. (b) Partial-volume related artifacts seen on synthetic FLAIR images.