1771
Improved combination of multi-inversion time images for fluid and white matter suppression1Center for Neuroscience and Regenerative Medicine, Henry M. Jackson Foundation, Bethesda, MD, United States, 2National Institutes of Health, Bethesda, MD, United States, 3Henry M. Jackson Foundation, Bethesda, MD, United States
Synopsis
Fluid and White Matter Suppression (FLAWS) MRI uses two inversion times within a T1-weighted magnetization-prepared gradient echo sequence to enhance visualization of gray matter structures within the brain. FLAWS is based on the MP2RAGE sequence, but with different inversion times, followed by a voxel-wise minimum. In this work, we demonstrate that a variation of the regularized, ratio combination approach of MP2RAGE yields superior gray matter contrast-to-noise and intensity uniformity compared to the originally proposed FLAWS MRI combination based on minimum intensity.
Introduction
Fluid and White Matter Suppression (FLAWS) MRI uses two inversion times within a T1-weighted magnetization-prepared gradient echo sequence to enhance visualization of gray matter structures within the brain [1]. FLAWS is based on the Magnetization Prepared 2 Rapid Acquisition Gradient Echoes (MP2RAGE) sequence [2,3], but uses a voxel-wise minimum to combine the images. Improved subcortical gray matter contrast from FLAWS has been shown to facilitate planning for deep brain stimulation [1,4], while its improved cortical contrast has shown promise in the detection of epileptogenic foci [5]. A drawback of the minimum combination approach, however, is that by always taking the lower of two signal values, signal-to-noise ratio is compromised. Furthermore, robustness to field inhomogeneity artifacts, a hallmark of the original MP2RAGE sequence, is lost. We show here that a variation of the standard MP2RAGE combination provides improved contrast-to-noise and uniformity compared to the voxel-wise minimum proposed in [1], therefore offering superior visualization of both subcortical and cortical gray matter.Methods
FLAWS optimizes inversion times to suppress white matter in the first image, and cerebrospinal fluid (CSF) in the second image (see Figure 1). We implemented FLAWS on both a Philips Achieva 3T and a Siemens Biograph mMR. Four healthy subjects were scanned, one subject on both Philips and Siemens scanners, and the other three subjects only on Philips. The Philips scans were either 1mm isotropic or 0.75x0.75x0.8mm, with TR=5.5-6s, TE=2.51-2.7ms, FA=5, BW=310-347, TI1=422-423ms, and TI2=1113-1164ms. The Siemens scan was 1mm isotropic with TR=5s, TE=1.6ms, FA=5, BW=370, TI1=409ms, and TI2=1100ms. Acquisition time ranged from 9 mins 40 secs to 12 minutes for the higher resolution scans.Combination of the two images was performed in two ways. First, given the complex acquired images images GRETI1 and GRETI2 the standard FLAWS combination approach was computed as
$$S = \min(|\mathrm{GRE}_{\mathrm{TI1}}|,|\mathrm{GRE}_{\mathrm{TI2}}|)$$
where $$$S$$$ is the final combined image, suppressing both white matter (WM) and CSF. The second combination approach proposed here is a simple variation of the MP2RAGE equation [3], which is known to have the added benefit of removing B1 inhomogeneity artifacts:
$$S=\frac{-Re(\mathrm{GRE}^*_{\mathrm{TI1}}\mathrm{GRE}_{\mathrm{TI2}}) - \beta}{|\mathrm{GRE}^*_{\mathrm{TI1}}|^2+|\mathrm{GRE}^*_{\mathrm{TI2}}|^2+2\beta}$$
where $$$\beta$$$ is a regularization parameter to reduce background noise. This equation is identical to that in [3] except for a sign change in the numerator. The sign change was required to invert the intensity scale, allowing gray matter to be brighter than WM and CSF (see Figure 2). In this work, $$$\beta$$$ was automatically set to be equal to the square of 10% of the 99th percentile maximum of $$$|\mathrm{GRE}^*_{\mathrm{TI1}}|$$$ for all scans.
To quantitatively evaluate the contrast-to-noise ratio (CNR) of the different combination approaches, the FreeSurfer segmentation algorithm [6] was applied to $$$\mathrm{GRE}^*_{\mathrm{TI1}}$$$, with labels merged to define cortical gray matter (CGM), subcortical gray matter (SGM), WM, and ventricular CSF. Let $$$T_{FG}=\{CGM,SGM\}$$$ and $$$T_{BG}=\{WM,CSF\}$$$. For each tissue class in $$$T_{FG}$$$ and $$$T_{BG}$$$, CNR was defined as
$$\mathrm{CNR}=\frac{|\mu(T_{FG}-\mu(T_{BG})|}{\sigma(T'_{FG})}$$
where $$$\mu$$$ represents the mean, and $$$\sigma$$$ represents standard deviation. The notation $$$T'_{FG}$$$ indicates a one voxel erosion was applied to the region to reduce partial volume effects. Note that perfect spatial registration exists between all images. To quantitatively evaluate uniformity, N4ITK [7] was applied to both the minimum combined image and the proposed combination, and the bias field within the brain was extracted.
Results
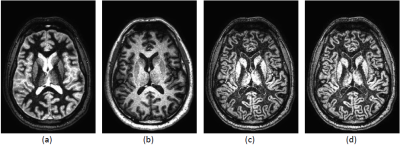
Figure 1 shows the FLAWS MRI result using the standard minimum combination approach from a healthy volunteer acquired on the Philips scanner. As expected, white matter is well suppressed in the first inversion time image, and CSF is suppressed in the second inversion time image. Intensity variations due to B1 inhomogeneity are evident in Figure 1(c), however, with some shading apparent at anterior portions of the cortex, and brighter intensities towards the center. These artifacts are reasonably minimized using N4ITK, as shown in Figure 1(d).Figure 2(a) shows the results of applying the standard MP2RAGE equation to the same data set. When inverted (Figure 2(b)) the gray matter becomes bright. Background noise is removed by incorporating the regularization parameter in the combination equation (Figure 2(c)). The range of intensity values in this approach is -0.5 to 0.5. By applying a window and level setting that normalizes the display range to 0 to 0.5, Figure 2(d) is obtained, which closely resembles the minimum combination approach. However, the gray matter structures gain a smoother appearance, and possess superior uniformity.
Quantitatively, CNR was consistently equivalent or higher using the proposed approach. Average CNR across the five scans for CGM to WM was 1.98 for the proposed vs 1.39 for the minimum, 5.43 vs 1.78 for CGM to CSF, 3.16 vs 2.45 for SGM to WM, and 6.94 to 2.81 for SGM to CSF. In terms of bias fields measured with N4ITK, the proposed approach ranged on average from -9.1% to 5.6% away with a coefficient of variation of 2.7%. The minimum approach ranged from -17.1% to 25.1%, with a coefficient of variation of 7.93%, indicating much larger fluctuations in intensity due to B1 inhomogeneity.
Conclusion
The proposed combination approach provides superior visualization of gray matter compared to the original voxel-wise minimum, making it more suitable for clinical research applications targeting both subcortical and cortical structures.Acknowledgements
This work was supported by the Intramural Research Program in the Clinical Center of the National Institutes of Health and the Department of Defense in the Center for Neuroscience and Regenerative Medicine.References
1. Tanner, M, Gambarota, G, Kober, T, Krueger, G, Erritzoe, D, Marques, JP, Newbould, R (2012). Fluid and white matter suppression with the MP2RAGE sequence. J Magn Reson Imaging, 35, 5:1063-70.
2. Marques, JP, Kober, T, Krueger, G, van der Zwaag, W, Van de Moortele, PF, Gruetter, R (2010). MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage, 49, 2:1271-81.
3. O'Brien, KR, Kober, T, Hagmann, P, Maeder, P, Marques, J, Lazeyras, F, Krueger, G, Roche, A (2014). Robust T1-weighted structural brain imaging and morphometry at 7T using MP2RAGE. PLoS ONE, 9, 6:e99676.
4. Beaumont, J, Saint-Jalmes, H, Acosta, O, Kober, T, Tanner, M, Ferré, JC, Salvado, O, Fripp, J, Gambarota, G (2019). Multi T1-weighted contrast MRI with fluid and white matter suppression at 1.5 T. Magn Reson Imaging, 63:217-225.
5. Chen, X, Qian, T, Kober, T, Zhang, G, Ren, Z, Yu, T, Piao, Y, Chen, N, Li, K (2018). Gray-matter-specific MR imaging improves the detection of epileptogenic zones in focal cortical dysplasia: A new sequence called fluid and white matter suppression (FLAWS). Neuroimage Clin, 20:388-397.
6. Fischl, B (2012). FreeSurfer. Neuroimage, 62, 2:774-81.
7. Tustison, NJ, Avants, BB, Cook, PA, Zheng, Y, Egan, A, Yushkevich, PA, Gee, JC (2010). N4ITK: improved N3 bias correction. IEEE Trans Med Imaging, 29, 6:1310-20.
Figures