1768
Evaluation of Compressed Sensing Approaches for Rapid Anatomical Imaging of Patients with Brain Tumors1Radiology & Biomedical Imaging, UCSF, San Francisco, CA, United States, 2Berkeley Institute for Data Science, UC Berkeley, Berkeley, CA, United States, 3GE Healthcare, Menlo Park, CA, United States
Synopsis
Compressed Sensing with parallel imaging can allow for an accelerated anatomical imaging protocol for imaging patients with brain tumors, saving ~10 minutes of scan time over standard clinical protocols when incorporated into 3D T2-FLAIR, and 3D T1-weighted pre- and post- contrast imaging. We first determined the optimal undersampling pattern and acceleration for CS T1-weighted IR-SPGR sequences and then evaluated the quality of the images and lesion definition in patients with brain tumors. This shortened anatomical imaging protocol has the potential to reduce the frequency of motion artifacts and can allow time for more advanced, therapy specific imaging to be acquired.
Introduction
Under current practice, the anatomical imaging portion of clinical brain tumor MR imaging protocols typically takes approximately 30 minutes to acquire conventional 3D pre- and post-contrast T1-weighted IR-SPGR, T2-weighted FSE, and T2-weighted FLAIR sequences with 2-fold accelerations using parallel imaging. Reduction of this time by 5-10 minutes could greatly improve clinical workflow by allowing time for therapy-specific advanced imaging beyond standard diffusion and perfusion to be obtained within the typical 45-minute clinical examination. To achieve this, we explored different view-ordering schemes with compressed sensing for pre- and post-contrast 3D gradient-echo based T1 imaging and evaluated a 3D CUBE T2 FLAIR1 with compressed sensing (CS)2 and in-plane ARC3 reconstruction to shorten the two longest sequences in our anatomical protocol. The first goal of this study was to determine the optimal undersampling pattern and acceleration for CS T1-weighted IR-SPGR sequences in 4 healthy volunteers and 4 patients with brain tumors. We then evaluated the feasibility of using the optimally accelerated T1-weighted IR-SPGR images and previously optimized CS T2 FLAIR images in clinical practice by comparing the image quality and volumes of segmented contrast-enhancing and T2-hyperintensity lesions between the standard and 4-fold accelerated images acquired in the same patient.Methods
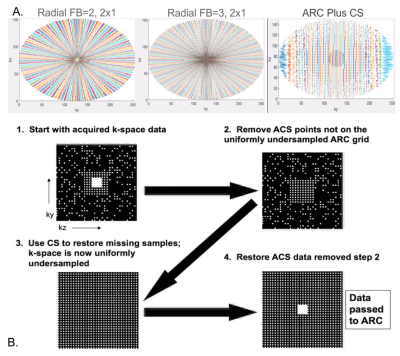
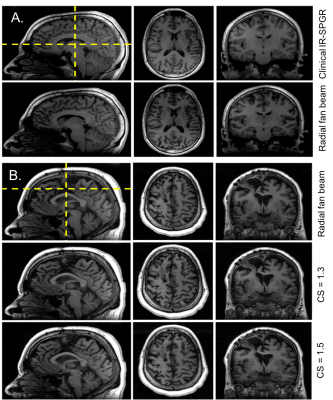
All scans took place on a GE Premier 3T scanner equipped with 48-channel phased-array head coil. Four healthy volunteers were scanned with a 3D T1 sequence (BRAVO) that consisted of either a fully-sampled Cartesian with linear view ordering scheme or segmented k-space acquisition approach where acquisition segments were radially distributed in ky-kz space (radial fan beam view ordering) as shown in Figure 1A. Other sequence parameters were TI/TE/TR=450/3.1/8.1ms, flip angle=12o, BW=50, FOV=25.6cm, matrix size=256x256x172, for 1mm3 isotropic resolution. A 2-fold acceleration with ARC was applied in-plane, followed by CS reconstruction as shown in Figure 1B. Likert-scale ratings (1-5) of overall image quality and, when applicable, lesion conspicuity, were evaluated by a neuro-radiologist who was blinded to the type of scan and was presented the different fully and under-sampled images in a random order.After initial optimization, 6 patients with gliomas (grades II-IV) were then scanned with a protocol consisting of the 3D T2 FLAIR sequence (TI/TE/TR=1725/105/6002ms, FOV=25.6cm, matrix size=256x256, 2-fold in-plane ARC acceleration, 1x1x1.2mm3 resolution, 6min) with and without an additional CS acceleration of 1.25, 4.3min) and post-contrast imaging with a conventional ARC-accelerated IR-SPGR sequence (4:10min) or the radial fan beam T1-weighted IR-SPGR with CS acceleration factor=1.3. For the post-contrast imaging, half of the scans were performed with the CS-accelerated acquisition first, while the order was switched in the other half of patients. Metrics for comparison between the same image contrasts included volumes of T2 hyperintensity and contrast-enhancing lesions in addition to the Likert ratings described above. Statistical analyses included correlations between the volumes of each paired acquisition as well as Wilcoxon signed-rank tests to evaluate any significant differences in Likert ratings between the accelerated and conventional scans.
Results
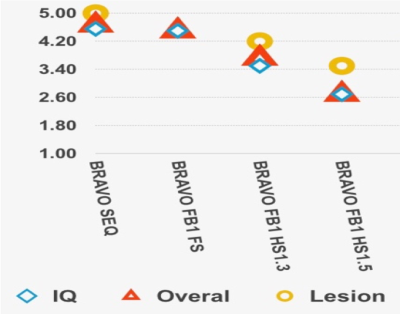
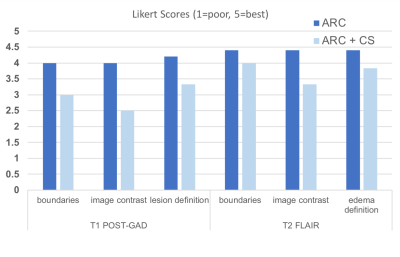
Optimization of CS T1-weighted images with radial fan beam view order: Figure 2 shows examples of different images for a patient scanned with different CS acceleration factors. Likert ratings comparing the image quality and lesion conspicuity are plotted in Figure 3. Overall image quality was highest for the radial fan beam sampling pattern. Although a slight reduction in quality was observed with increasing CS factor from 1 to 1.3, the nearly 4-fold reduction of scan time from 6:52 minutes to 1:43 outweighs the slight decrease in quality. Further increasing the CS factor to 1.5 decreases the quality by another 20% while only saving 12 seconds. We found that given the balance between additional time saved and image quality, a CS acceleration factor of 1.3 within the fan beam sampling combined with a 2-fold acceleration with ARC in-plane phase encode had the optimal image quality for reduction in scan time.Lesion assessment with accelerated imaging protocol: Figure 4A compares the T2 hyperintensity lesion volumes and contrast-enhancing lesion volumes (when present) between the standard clinical and CS-accelerated acquisitions. An example of each for a representative patient is shown in Figure 4B, where the CS+ARC scans were able to capture both the full extent of a large T2 lesion and equally visualize a very small region contrast enhancement. Lesion volumes were highly linear with a slope of 1 (r=.99; p<0.00001) and no statistically significant difference was observed in lesion volumes between the two acquisitions (p=0.9). Although decreasing trends in lesion definition, internal contrast, and boundaries were apparent with the ARC+CS acquisitions, none of these differences were statistically significant (p=.125; Figure 5). In two patients, the ARC+CS images received higher ratings than the ARC images alone because of motion artifacts present in the longer scans.
Conclusions
Compressed Sensing with ARC can allow for an accelerated anatomical imaging protocol for imaging patients with brain tumors, saving ~5 minutes over ARC-accelerated protocols and nearly 10 minutes of scan time over standard clinical protocols when incorporated into 3D T2 FLAIR, and 3D T1-weighted pre- and post-contrast imaging. This shortened anatomical imaging protocol has the potential to reduce the frequency of motion artifacts and can allow time for more advanced, therapy-specific imaging to be acquired in these patients.Acknowledgements
Funding support for this research was provided by GE Healthcare.References
1. Busse RF, Brau ACS, Vu A, et al. Effects of Refocusing Flip Angle Modulation and View Ordering in 3D Fast Spin Echo Rowley Magnetic Resonance in Medicine 60:640–649 (2008)
2. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007 Dec;58(6):1182-95.
3. Brau AC, Beatty PJ, Skare S, Bammer R.Comparison of reconstruction accuracy and efficiency among autocalibrating data-driven parallel imaging methods. Magn Reson Med. 2008 Feb;59(2):382-95.
Figures