1766
Initial feasibility of a multi-band PSF-mapping based, reverse-gradient approach with geometric distortion correction for whole-brain fMRI1Department of Radiology, Mayo Clinic, Rochester, MN, United States, 2Department of Physiology, College of Medicine, Hanyang University, Seoul, Korea, Republic of
Synopsis
A point-spread-function mapping-based reverse-gradient approach was demonstrated as a viable method to correct severe susceptibility artifacts for deep-brain-stimulation fMRI in a pig model, but at the cost of reduced temporal resolution. Interleaved acquisition of the echo-planar-imaging was used with opposite phase-encoding polarities. In this work, feasibility was evaluated in in-vivo resting-state fMRI reliability in high-susceptibility regions. To compensate for the reduced temporal resolution, multi-band imaging was used, and the improved reliability in highly susceptible regions was evaluated on both standard whole-body and high-performance compact 3T scanners.
Introduction
Gradient-echo echo-planar imaging (EPI) is the most commonly-used method for fMRI acquisition. It is prone to signal dropout and geometric distortion artifacts in regions of rapid susceptibility change, including the frontal and temporal lobes. A recent study [3] demonstrated that a PSF mapping-based reverse-gradient (PSF-RG) approach can minimize susceptibility artifacts in a pig’s brain for fMRI during deep-brain-stimulation. In this work, the effectiveness of PSF-RG approach was evaluated in the human brain for fMRI, especially in high susceptibility brain regions. Since a pair of interleaved EPI acquisitions with opposite phase-encoding gradient polarity were employed to minimize the signal dropout, the temporal resolution of the fMRI is reduced by half. However, this loss can be fully compensated for using a multi-band acceleration technique [4]. The method was tested on both a clinical whole-body 3T (WB3T) and a high-performance compact 3T (C3T) scanner [4-6].Methods
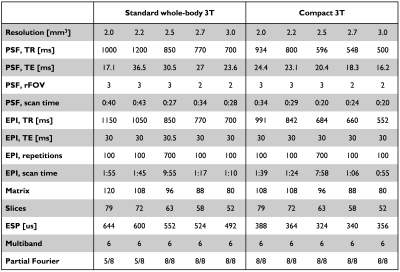
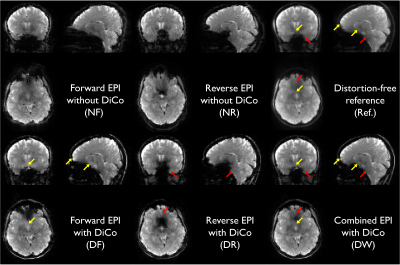
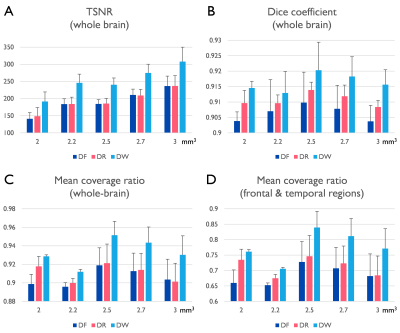
After informed consent, three subjects were scanned using a 32-channel coil (Nova medical, USA) on a clinical 3T scanner, GE 750 (GE Healthcare, Waukesha, WI). One of subjects was scanned on both the WB3T and the C3T. With the multi-band implementation, the PSF and the corresponding EPI fMRI pair with opposite PE polarity was acquired with 5 different isotropic resolutions (2.0, 2.2, 2.5, 2.7, and 3.0 mm). A relatively high multi-band factor of 6 was applied without in-plane acceleration. The imaging protocol details are provided in Table 1. After PSF mapping-based distortion correction [3,7], a total of five different variants of the EPI series including: forward and reverse phase-encoded EPIs without (NF and NR), and with distortion correction (DF and DR) and the weighted combination of the distortion-corrected EPI pair (DW) were obtained. To investigate the effectiveness of this approach for human fMRI studies, the signal recovery performance was evaluated both qualitatively and quantitatively. Quantitatively, temporal SNR (tSNR), mean coverage ratio, and the Dice similarity coefficient were computed. After co-registration between the functional and the anatomical images with rigid body transform using AFNI software [8], all functional data were interpolated to 2.0 mm resolution, and masked based on the temporal SNR map. Labeling of brain regions based on anatomical image was performed using FreeSurfer [9]. Both the Dice similarity coefficients (i.e., the Sorensen-Dice index) and the coverage ratio between EPI and anatomical volume, were evaluated. Finally, the results obtained on both the standard clinical and the high-performance compact 3T scanners were compared.Results and Discussion
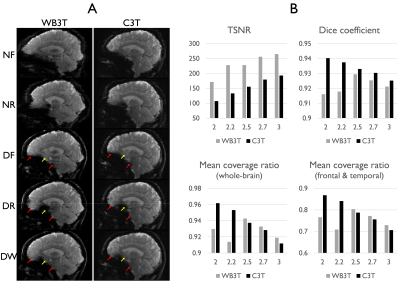
Severe susceptibility artifacts appeared as signal dropout and geometric distortions, especially in regions of temporal and frontal lobes of the human brain (Fig. 1). Signal dropout varied across the slices within a subject depending on the phase-encoding polarity. The geometric mismatch between the distortion-free reference and EPI without correction was significant in local areas. The complex pattern could easily mislead the interpretation of fMRI activations. With the proposed correction scheme, the distortion-corrected EPI pairs with opposite PE polarities were better geometrically matched with the distortion-free reference image. The signal dropout was reduced in the combined distortion-corrected EPI pairs (Fig. 1). The quantitative indices in the combined image are the best among all image datasets regardless of spatial resolution (Fig. 2). Temporal SNR tended to increase with the voxel size. Interestingly, high-resolution imaging offered a better coverage ratio on the C3T, which may be due to reduced signal dropouts associated with partial volume effects (Fig. 3B). In distinction, 2.5 mm resolution data excelled in the quantitative comparison on the WB3T (Figs. 2) since partial Fourier acquisition was required to keep the TE as 30 ms for high-resolution imaging (> 2.5 mm3) on the WB3T and leaded to signal dropout arising from the incorrect phase information [10] in the vendor reconstruction. The use of high-performance gradients greatly reduced the echo spacing on the C3T [11], which was advantageous to acquire 2.0 mm resolution data without partial Fourier acquisition (Table 1), and to reduce the level of distortion in EPI (Fig. 3A). Nevertheless, the PSF-RG scheme was still effective to further minimize the artifacts in local areas even on the C3T (Fig. 3A). With a relatively high multi-band factor of 6, the effective temporal resolution of the RG approach was 1.70 and 1.19 seconds, respectively on the WB3T and C3T, even at 2.5 mm isotropic resolution, whole-brain fMRI. Furthermore, the PSF scans required less than 30 seconds on both scanners (Table 1). Thus, the effective temporal resolution for the RG approach and the calibration time for distortion correction are quite practical for fMRI studies.Conclusion
This feasibility study demonstrates that the PSF-based RG approach can be beneficial to improve human fMRI in high-susceptibility areas, on both a standard whole-body and a high-performance compact 3T. In conjunction with the multi-band imaging, the temporal resolution of the RG approach and the calibration scan for distortion correction are practical for fMRI.Acknowledgements
This work was supported by NIH U01 EB024450 and NHI U01 EB026979.References
1. Visser M, Embleton KV, Jefferies E, Parker GJ, Ralph MA The inferior,
anterior temporal lobes and semantic memory clarified: novel evidence from
distortion-corrected fMRI. Neuropsychologia. 2010 May; 48(6):1689-96.
2. Devlin JT, Russell RP, Davis MH, Price CJ, Wilson J, Moss HE, Matthews
PM, Tyler LK Susceptibility-induced loss of signal: comparing PET and fMRI on a
semantic task. Neuroimage. 2000; 11:589-600.
3. In, M. H., Cho, S., Shu, Y., Min, H. K., Bernstein, M. A., Speck, O., et
al. Correction of metal-induced susceptibility artifacts for functional MRI
during deep brain stimulation. Neuroimage 2017;158:26–36. doi:
10.1016/j.neuroimage.2017.06.069
4. Lee S-K, Mathieu J-B, Graziani D, et al. Peripheral nerve stimulation
characteristics of an asymmetric head-only gradient coil compatible with a
high-channel-count receiver array. MRM 2016;76:1939–1950. doi:
10.1002/mrm.26044.
5.
Foo TK, Laskaris E, Vermilyea M,
Xu M, Thompson P, Conte G, Van Epps C, Immer C, Lee SK, Tan ET. Lightweight,
compact, and high-performance
3T MR system for imaging the brain and extremities. MRM 2018. doi: 10.1002/mrm.27175.
6.
Weavers PT, Shu Y, Tao S, Huston J 3rd, Lee
SK, Graziani D, Mathieu JB, Trzasko JD, Foo TK, Bernstein MA. Compact
three-tesla magnetic resonance imager with high-performance gradients passes
ACR image quality and acoustic noise tests. Med Phys 2016; 43:1259-64.
7. In, M. H.,
Posnansky, O., Beall, E. B., Lowe, M. J., & Speck, O. Distortion correction
in EPI using an extended PSF method with a reversed phase gradient approach.
PLoS One 2015;10(2), e0116320
8.
Cox, R.W., AFNI: software for
analysis and visualization of functional magnetic resonance neuroimages. Comput
Biomed Res, 1996. 29(3): p. 162-73.
9.
Reuter, M., et al.,
Within-subject template estimation for unbiased longitudinal image analysis.
Neuroimage, 2012. 61(4): p. 1402-18.
10. Zhang, X., Yacoub, E., & Hu, X. New strategy for reconstructing
partial‐Fourier imaging
data in functional MRI. Magn
Reson Imaging. 2001;46(5), 1045-1048.
11. Tan ET, Lee SK, Weavers PT, Graziani D, Piel JE, Shu Y, Huston J 3rd, Bernstein MA, Foo TK. J High slew-rate head-only gradient for improving distortion in echo planar imaging: Preliminary experience. Magn Reson Imaging. 2016 Sep;44(3):653-64. doi: 10.1002/jmri.25210.
Figures