1765
Simultaneous Brain/Spinal Cord fMRI reveals Subject-Level Activation Differences under Noxious Thermal Stimulus1Stanford University, Stanford, CA, United States
Synopsis
Functional activation within brain has been studied extensively via BOLD fMRI. Limiting investigation to brain alone provides a truncated view of the central nervous system as it does not capture information exchange between brain and spinal cord. Simultaneously imaging brain, brainstem, and cervical spine provides insight into pain modulation pathways because dorsal horn is the first synapse connecting periaqueductal gray with cortical pain regions. Sprenger & Finsterbusch (2015) have shown group-level functional connection of brain to spinal cord under noxious thermal stimulus. Here, we investigate subject-level differences in neural activity in brain-spinal cord.
Introduction
Functional activation within brain has been studied extensively via BOLD fMRI. Limiting investigation to brain alone provides a truncated view of the central nervous system as it does not capture information exchange between brain and spinal cord. Simultaneously imaging brain, brainstem, and cervical spine provides insight into pain modulation pathways because dorsal horn is the first synapse connecting periaqueductal gray with cortical pain regions1. Sprenger & Finsterbusch (2015) have shown group-level functional connection of brain to spinal cord under noxious thermal stimulus.1 Here, we investigate subject-level differences in neural activity in brain-spinal cord.Methods
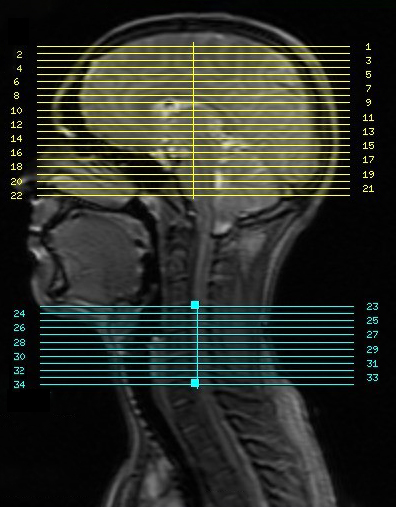
By pulse sequence designed & implemented under KSFoundation platform,2 and dynamic shimming reported in Islam & Law,3 one 7.5-minute scan per subject was collected at 3T (GE Discovery 750 scanner). BOLD fMRI sequence parameters: EPI GRAPPA R=2, flip angle=80°, brain/spinal cord FOV=22cm/8cm, 64x64 matrix, TE/TR=30ms/2s, #slices=34 (22 brain, 12 spine centered at C5 as seen in Fig.1), slice thickness/spacing=5/0mm. Thermal stimulation to induce activation at dermatome C6 delivered by 30x30mm2 thermode (TSA-II, Medoc) on right palm thenar eminence.Three healthy subjects participated. Prior to scan, each subject underwent a thermal calibration procedure to establish a temperature setpoint for perceived pain intensity 6 out of 10; on a scale 0 for no pain, 10 being unbearable pain. At scan time, each subject’s setpoint temperature was maintained during on-cycles (15s on/off) of their 7.5-minute scan. Physiological data was collected by respiratory belt and pulse oximeter.
Physiological noise is removed from brain and spinal cord images by RETROICOR.4 Images are corrected for slice timing and motion, after which, mean cervical spinal fluid and white matter signals are removed using custom software. Brain and spinal cord data are spatially smoothed separately: 5×5×5 mm3 FWHM Gaussian kernel for brain, 2×2×5mm3 FWHM Gaussian kernel for spine. Subject-level analysis is performed using FSL FEAT.5
Results
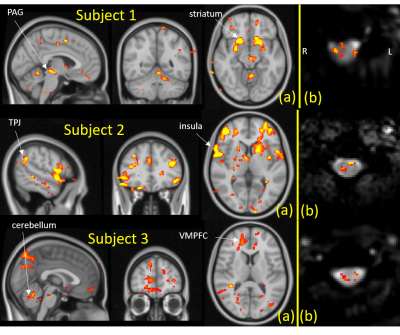
Figures 2 illustrates brain and spinal cord activation from Subjects 1-3. Spinal cord slices illustrated are from C6 segment which corresponds to dermatome of the stimulation site.6 BOLD response to noxious thermal stimulus is observed at right dorsal horn in all three subjects. In the brain, ventromedial prefrontal cortex, cerebellum, brain stem, and temporoparietal junction are among activated regions at threshold Z>1.7, common to all three subjects. But only subjects 1&2 have strong activation in striatum and insula whereas only subjects 2&3 show activation in anterior cingulate cortex.Conclusion
Our simultaneous brain/spinal cord pulse sequence reveals activation at subject level due to noxious thermal stimuli. While spinal segment C6 dorsal horn signal is common to all three subjects, intersubject brain activation is inconsistent. Dynamics of pain perception is part of the challenge to pain research. This observation highlights the intricate and dynamic nature of pain perception and processing and confirms the need for concurrent acquisition of brain and spinal cord fMRI.Discussion
Previous studies have demonstrated that various thermal stimulation levels vary BOLD activation both in brain and spinal cord.7,8 The higher the temperature, broadly speaking, the stronger the activation. One possible explanation of individual differences in brain activation is that two subjects reported a change in perceived pain intensity during experiments, with respect to their calibrated setpoint temperature, despite the fact that their setpoint was administered during scan. Subject 1 reported that pain intensity increased to 9/10 whereas Subject 3’s perception lowered to 4/10 toward the end of their 7.5-minute scan. Subject 2 reported that pain intensity remained 6/10 throughout scan.Acknowledgements
No acknowledgement found.References
1. Sprenger C, Finsterbusch J, & Buchel C. Spinal Cord–Midbrain Functional Connectivity Is Related to Perceived Pain Intensity: A Combined Spino-Cortical fMRI Study. The Journal of Neuroscience, March 11, 2015; 35(10):4248 – 4257.
2. http://ksfoundationepic.org/
3. Islam H, Law CSW, Weber KA, Mackey SC, Glover GH. Dynamic per slice shimming for simultaneous brain and spinal cord fMRI. Magn Reson Med. 2019 Feb;81(2):825-838.
4. Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000 Jul;44(1):162-7.
5. Woolrich, M. W., Ripley, B. D., Brady, M., & Smith, S. M. (2001). Temporal Autocorrelation in Univariate Linear Modeling of FMRI Data. NeuroImage, 14(6), 1370–1386.
6. Goto N, Otsuka N. Development and anatomy of the spinal cord. Neuropathology 1997 Mar;17(1):25–31.
7. Kutch JJ, Labus JS, Harris RE, Martucci KT, Farmer MA, Fenske S, Fling C, Ichesco E, Peltier S, Petre B, Guo W, Hou X, Stephens AJ, Mullins C, Clauw DJ, Mackey SC, Apkarian AV, Landis JR, Mayer EA, Network MR. Resting-state functional connectivity predicts longitudinal pain symptom change in urologic chronic pelvic pain syndrome: a MAPP network study. Pain. 2017;158(6):1069-1082.
8. Weber KA, 2nd, Chen Y, Wang X, Kahnt T, Parrish TB. Functional magnetic resonance imaging of the cervical spinal cord during thermal stimulation across consecutive runs. Neuroimage. 2016;143:267-279.
Figures