1763
Isotropic High-Resolution Brain T1rho Mapping with 3D FLAIR MAPSS at 3T1Gruss Magnetic Resonance Research Center, Department of Radiology, Albert Einstein College of Medicine and Montefiore Medical Center, Bronx, NY, United States, 2Philips Healthcare, Andover, MA, United States, 3Program of Advanced Musculoskeletal Imaging (PAMI), Cleveland Clinic, Cleveland, OH, United States
Synopsis
Quantitative T1rho (T1ρ) mapping MRI has recently gained wider clinical/research application in human brain imaging. However, high resolution 3D brain T1rho mapping on clinical scanners is still time consuming with compromised quantitative accuracy due to image blurring or artifacts. We propose a 3D FLAIR MAPSS T1rho imaging scheme, which combines CSF-suppression with 3D MAPSS sequence for improved quantitative accuracy in T1rho mapping. Phantom, volunteer, and patient studies validated its sensitivity and reliability for isotropic high resolution of voxel size (1.3mm)3 quantitative mapping using continuous-wave or adiabatic RF pulse T1rho preparation, acquired within clinically acceptable scan duration at 3T.
INTRODUCTION
Quantitative T1rho (T1ρ) mapping MRI has been shown to be sensitive to pH, macromolecule density, and metabolic dynamics in the brain and have gained wide applications in human brain conditions such as multiple sclerosis,1 bipolar,2 tumor,3 and stroke.4 Ideally, quantitative T1rho mapping for the brain has isotropic high spatial resolution 3D whole brain coverage and high measurement accuracy and reliability, all acquired within reasonable scan duration. However, the scan duration for high resolution 3D T1rho mapping is particularly lengthened due to high SAR induced by long spin-lock RF pulses needed by relatively long T1rho of brain GM/WM tissues, and multiple spin-lock times needed for curve fitting. In addition, the accuracy and reliability of the T1rho measurements are greatly compromised by the image blurring/ghosting due to transient or relaxation decay effects by multiple shared acquisitions per preparation in segmented 3D GRE or FSE sequences. Artifacts and partial volume effects originated from the CSF (which has more than 10-fold higher T1rho than those of the brain tissues) further contaminate the quantitative maps. The purpose of this work is to present an isotropic high-resolution 3D FLAIR MAPSS T1rho sequence, re-optimized for brain based on a synovial fluid suppressed 3D T1rho sequence recently developed to achieve fast and accurate quantitative mapping for human knee cartilage.5 This 3D FLAIR MAPSS technique was combined with either traditional continuous-wave T1rho (cw-T1rho), or more recent adiabatic T1rho (adiab-T1rho) preparation modules and was tested in phantom, volunteer, and patient studies to validate its effectiveness for high resolution and accurate quantitative mapping of the brain.METHODS
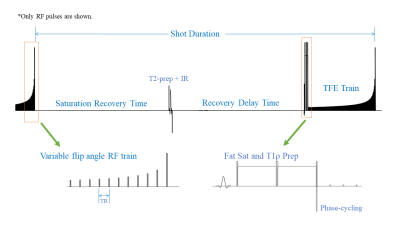
The 3D FLAIR MAPSS sequence scheme is shown in Fig.1. Bloch simulation was performed to optimize sequence timing and shot duration of 5000ms was chosen to achieve maximum SNR efficiency for brain WM. In each shot, a T2-preparation module (with TE=140ms) followed by an inversion recovery RF pulse was employed to selectively invert CSF and saturate brain tissues.6 After optimized TI delay of 1600ms to null CSF, a MAPSS sequence scheme was used to achieve fast and accurate T1rho imaging.7 All imaging was performed on a 3T Philips Ingenia Elition MR scanner with a 32-channel head coil. The cw-T1rho preparation module had 90°-TSL/2-180°-TSL/2-90° RF pulse train, with spin lock frequency=500Hz and TSL=0, 50, 60ms. The adiab-T1rho preparation module had adiabatic HS-8 pulse trains with TSL=4, 52, 68ms. 3D sagittal volumetric imaging was performed with the following scan parameters: FOV=230/230/180mm, acquisition voxel size=(1.3mm)3, TR/TE=5.7/2.7ms, echo train length of 180 with centric profile ordering, readout bandwidth =48kHz, and compressed SENSE factor=9. As previously proposed,7 phase-cycling, and variable flip angle train as described in were employed to minimize imaging blurring/ghosting along the phase encode directions to maximize quantitative accuracy. The scan duration was about 6:45 min for both the cw-T1rho and the adiab-T1rho scans. In the phantom study, the proposed cw-T1rho 3D FLAIR MAPSS was compared to an earlier validated 3D MAPSS cw-T1rho sequence (with TSL=0, 20, 40, 60) using the same phantom of the report.8RESULTS
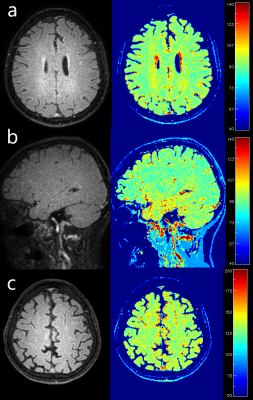
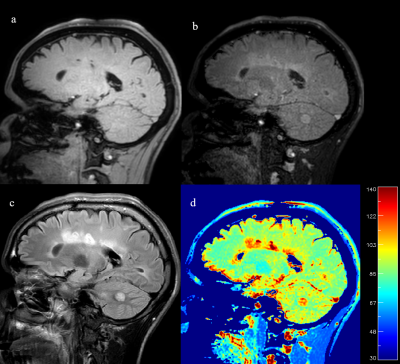
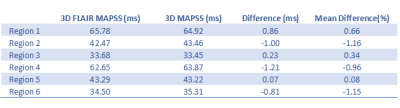
Phantom regions of interest measurement results were highly consistent between the proposed 3D FLAIR MAPSS sequence and the reference 3D MAPSS sequence (see Table 1). The mean difference of the T1rho measurements were all under 1.2% with average mean difference -0.4%. In human studies, both cw- and adib-T1rho 3D FLAIR MAPSS sequences achieved effective CSF suppression, with WM-to-CSF contrast-to-noise ratio consistently larger than 10. No CSF-related artifacts could be identified. Fig.2 shows representative 3D FLAIR MAPSS T1rho imaging results from a 66-yo volunteer without prior known WM conditions. It is shown that both cw- and adiab-T1rho sequences had high resolution and sensitivity to detect small demyelinating lesions. Fig.3 shows representative T1rho imaging results from an MS patient, compared with the corresponding 2D FLAIR image. All hyperintense lesions shown on FLAIR had elevated T1rho values, validating the sensitivity of the tested sequence.DISCUSSION
Commonly used segmented GRE or FSE brain T1rho mapping sequences are flawed since quantitative accuracy is compromised by ghosting/blurring originated from transient or relaxation decay effects. It is more problematic when high spatial resolution is needed. 3D MAPSS T1rho technique is a robust imaging technique specifically proposed to mitigate this problem.7 The CSF signal, if not suppressed, can be another major source of uncertainty in brain T1rho mapping. High CSF signal can compromise quantitative accuracy through partial volume effects in addition to severe blurring/ghosting. Furthermore, CSF-induced artifacts can spread to non-neighboring brain voxels when k-space under-sampling techniques (e.g., parallel imaging and compressed sensing) are employed. These fast imaging techniques are indispensable to reduce total scan duration in high resolution 3D T1rho mapping. It is shown in this work that the proposed 3D FLAIR MAPSS scheme can be used as a general solution for 3D cw-T1rho and adiab-T1rho mapping techniques for high resolution quantitative brain imaging. With this, high-resolution 3D T1rho mapping without CSF signal contamination can be obtained on clinical 3T scanners within acceptable scan duration.CONCLUSION
High resolution, CSF-suppressed, whole brain T1rho mapping with isotropic voxel size can be achieved within clinically acceptable scan duration using 3D FLAIR MAPSS sequences. This will help improve its quantitative accuracy, expand its research applications, and facilitate its wider clinical adoption.Acknowledgements
No acknowledgement found.References
1. Borthakur A, Sochor M, Davatzikos C, et al. T1rho MRI of Alzheimer's disease. Neuroimage. 2008;41(4):1199-1205.
2. Johnson CP, Follmer RL, Oguz I, et al. Brain abnormalities in bipolar disorder detected by quantitative T1rho mapping. Mol Psychiatry. 2015;20(2):201-206.
3. Villanueva-Meyer JE, Barajas RF, Jr., Mabray MC, et al. Differentiation of brain tumor-related edema based on 3D T1rho imaging. Eur J Radiol. 2017;91:88-92.
4. Tan Y, Xu J, Chen R, et al. Use of T1 relaxation time in rotating frame (T1 rho) and apparent diffusion coefficient to estimate cerebral stroke evolution. J Magn Reson Imaging. 2018;48(5):1247-1254.
5. Peng Q, Wu C, Li X, et al. Improved 3D T1rho and T2 Mapping with Synovial Fluid Suppression for the Knee Cartilage on 3T. Proc Intl Soc Mag Reson Med 24. 2018:5056.
6. Wong EC, Liu TT, Luh WM, et al. T(1) and T(2) selective method for improved SNR in CSF-attenuated imaging: T(2)-FLAIR. Magn Reson Med. 2001;45(3):529-532.
7. Li X, Han ET, Busse RF, et al. In vivo T(1rho) mapping in cartilage using 3D magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots (3D MAPSS). Magn Reson Med. 2008;59(2):298-307.
8. Kim J, Mamoto K, Lartey R, et al. Multi-vendor multi-site T1ρ and T2 quantification of knee cartilage. Proc Intl Soc Mag Reson Med 25. 2019:0127.
Figures