1759
Postmortem Whole-Brain MP2RAGE Optimization at 3T: A New Imaging Window into Multiple Sclerosis Cortical Pathology1Translational Imaging in Neurology (ThINk) Basel, Department of Medicine and Biomedical Engineering, University Hospital Basel and University of Basel, Basel, Switzerland, 2Neurologic Clinic and Policlinic, Departments of Medicine, Clinical Research and Biomedical Engineering, University Hospital Basel and University of Basel, Basel, Switzerland, 3Radiological Physics, Department of Radiology, University Hospital Basel, Basel, Switzerland, 4Institute of Neuropathology, University Medical Center Göttingen, Göttingen, Germany, 5Advanced Clinical Imaging Technology, Siemens Healthcare, Lausanne, Switzerland, 6Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 7LTS5, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 8Department of Cognitive Neurology, MR-Research in Neurology and Psychiatry, University Medical Center Göttingen, Göttingen, Germany
Synopsis
The MP2RAGE sequence provides fast volumetric T1 weighted MR imaging and offers the possibility to reconstruct quantitative T1 maps. Therefore, it is frequently applied for studying Multiple Sclerosis pathologies in recent years. The present work investigates and explains necessary protocol changes for applying MP2RAGE in fixated human brain acquisitions. Using the established protocol, it is shown that strong soft tissue contrast is reinstated and quantitative T1 values can be derived for normal appearing gray matter and lesions. Based on the feasibility of using long scan times, the isotropic resolution of the MP2RAGE could be even increased to 0.75mm.
Introduction
The MP2RAGE sequence is an advancement of the magnetization prepared rapid gradient echo (MPRAGE1) sequence, which provides fast volumetric T1 weighted MR imaging with minimized sensitivity to excitation field, reception bias field, proton density, and T2* contrast2. In recent years, MP2RAGE acquisitions have been applied in clinical research to assess cortical and white matter pathology in Multiple Sclerosis (MS) patients both at 3T and 7T MRI3,4.In this work, obligatory parameter changes to harness the MP2RAGE capabilities for postmortem fixated whole-brain MR imaging at 3T field strength are investigated. The resulting optimized protocol was employed for the MRI of three fixated brains of patients who had suffered from MS.
Methods
The brain of three patients with secondary progressive MS were fixated in 10% formalin 24h to 48h after autopsy. For MRI acquisition, the brains were positioned in a dome-shaped container as depicted in 5-7 and immersed in a fluorinated oil (i.e. Fomblin). Air bubbles were aspirated through the spout of the container through a vacuum pump. All acquisitions were performed with a 3T wholebody MR system (Prismafit, Siemens Healthcare, Erlangen, Germany) using a 20-channel head coil.For a successful MP2RAGE implementation, as a minimum requirement, the sampling conditions must be adapted to the present T1 tissue relaxation times. A pre-assessment showed that the tissue T1 relaxation times can be as low as approx. 200ms in the measured brains at 3T. This observation posed a challenge for two reasons: (1) Low T1 relaxation times lead to fast signal changes during sampling, which eventually have a detrimental effect on the point spread function PSF (“T1 blurring”). (2) Short inversion times TI1 are needed for generating optimal brain tissue contrast. However, sub-millimeter spatial resolutions for the detection of small intracortical lesions are desired, which generally necessitate even longer readout times.
As a result, the following acquisition protocol was developed that addresses the requirements of this specific application: FOV=192x138x156mm3, a sagittal 3D slab of full isotropic 0.75mm resolution, 2D phase inside-3D phase outside-encoding scheme (“rotated”), parallel acquisition (GRAPPA) factor=3, ACS lines=32, bandwidth=700Hz/Pixel, ESP=4.2ms, TR=2700ms, TI1=194ms, TI2=2500ms, flip angles 6° and 7°, respectively, signal averaging=20, total acquisition time=3h 07min 12sec.
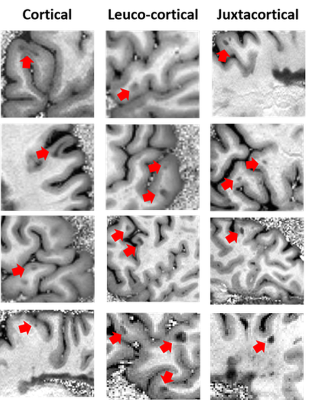
MS lesions (intracortical, leuco-cortical and juxtacortical) were identified by two independent readers on MP2RAGE uniform images.
Results
The developed solution uses quite unusual protocol settings for a MP2RAGE sequence: a high readout bandwidth of 700Hz/Pixel (instead of ca. 240Hz/Pixel) combined with a higher GRAPPA factor and a minimal number of ACS lines ensures ‘short’ readout modules, despite the sub-millimeter resolution. As a drawback, it results in a considerable loss of SNR that must be compensated by signal averaging. Additionally, the phase encoding loops were swapped to exploit the smaller acquisition dimension in phase-2D. Both recipes allow a considerable reduction of TI1 by approx. 270ms each for the given setup. Furthermore, the flip angles were raised by 2° each. As a benefit of the short T1s, TR could be reduced to the minimum, shortening the acquisition time without sacrificing signal.Figure 1 shows a comparison of two postmortem acquisitions, one with a standard 1mm isotropic MP2RAGE set up for in vivo conditions; and one with the developed 0.75mm isotropic postmortem scheme.
Figure 2 shows examples of intracortical lesions (i.e. lesions within the cortical layers like band-like lesions and small punctiform lesions), leuco-cortical lesions (i.e. lesions affecting both the cortex and the underlying white matter) and juxtacortical lesions (i.e. lesions that are affecting the superficial white matter/u-fibers but not the cortex), which were easily visualized by the two expert readers in the three brains.
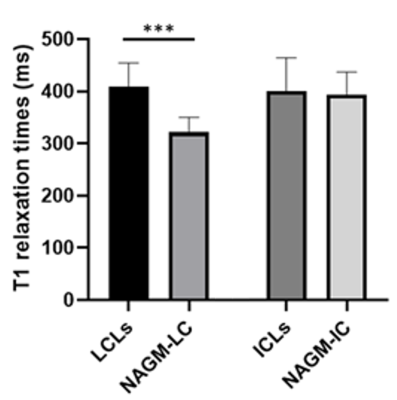
Figure 3 shows T1 relaxation times in intracortical, leuco-cortical and juxtacortical lesions as well as in the corresponding normal-appearing tissues. Coherent with MP2RAGE measurements obtained in living patients at 3T MRI3, T1 relaxation times are significantly longer in leuco-cortical lesions compared to the corresponding normal appearing regions, but there is no significant difference between cortical lesions and normal-appearing GM.
Discussion and Conclusion
This work shows the feasibility to acquire postmortem MP2RAGE in a fixated brain with an optimized contrast to detect focal cortical, leuco-cortical and juxtacortical MS lesions.Identifying lesions within and near the cortex is extremely challenging due to their small size and the presence of partial-volume voxels at the border between the cortex and the underlying white matter in clinical images3,4. MP2RAGE has shown an advantage in detecting lesions in the cortical ribbon and nearby in living patients, mainly thanks to its sharp contrast between white and grey matter. Moreover, MP2RAGE provides T1 relaxometry maps that help characterizing the lesions’ microstructure3,4.
Postmortem MP2RAGE may be beneficial for several reasons including the possibility to achieve higher spatial resolution than in in vivo scans and to compare imaging results with histopathological analysis. Neuropathological assessment is especially challenging for cortical lesions because areas of focal damage involving the cortex are often missed by macroscopic examinations. MP2RAGE will provide therefore a new window into cortical MS pathology to better identify areas that will undergo histopathological analysis. In addition, it provides microstructural measures that potentially relates to damage and repair phenomena in MS lesions and that may be translated into clinical assessments.
Acknowledgements
This work was funded by the Swiss National Funds PZ00P3_154508, PZ00P3_131914 and PP00P3_176984.References
1. Mugler JP 3rd, Brookeman JR. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE). Magn Reson Med 1990;15(1):152-7.
2. Marques JP, Kober T, Krueger G, et al. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage 2010;49(2):1271-81.
3. Kober T, Granziera C, Ribes D, et al. MP2RAGE multiple sclerosis magnetic resonance imaging at 3 T. Invest Radiol 2012;47:346-52.
4. Beck ES, Sati P, Sethi V, et al. Improved Visualization of Cortical Lesions in Multiple Sclerosis Using 7T MP2RAGE. AJNR Am J Neuroradiol 2018;39(3):459-466.
5. Luciano NJ, Sati P, Nair G, et al. Utilizing 3D Printing Technology to Merge MRI with Histology: A Protocol for Brain Sectioning. J Vis Exp 2016 Dec 6;(118).
6. Absinta M, Nair G, Filippi M, et al. Postmortem Magnetic Resonance Imaging to Guide the Pathologic Cut: Individualized, 3-Dimensionally Printed Cutting Boxes for Fixed Brains. J Neuropathol Exp Neurol. 2014;73(8):780-8.
7. Griffin AD, Turtzo C, Parikh G, et al. Traumatic microbleeds suggest vascular injury and predict disability in traumatic brain injury. Brain 2019;142(11):3550-3564.
Figures
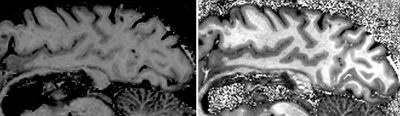
Figure 1: (Left) Sagittal slice from a 1mm isotropic MP2RAGE in vivo protocol acquired in a postmortem fixated brain (most relevant parameters: TI1=700ms, TI2=2500ms, TR=5000ms, bandwidth=240Hx/Pixel, α1=4°, α2=5°).
(Right) Sagittal slice of the developed 0.75mm isotropic MP2RAGE protocol dedicated for 3T postmortem, fixated brain acquisitions (for direct comparison: TI1=194ms, TI2=2500ms, TR=2700ms, bandwidth=700Hx/Pixel, α1=6°, α2=7°).