1758
Try this at home: Ex vivo Human Brain at 200 Micron Resolution with a Simple Set Up.1Cleveland Clinic Imaging Institute, CLEVELAND, OH, United States
Synopsis
Postmortem, whole-brain MRI provides an opportunity to investigate anatomy at spatial resolution approaching that of histology without the distortions and limited coverage inherent to histology. Previous work with ex vivo imaging has been done using a sophisticated process with additional steps, such as a custom-built vibration apparatus to remove air bubbles, proton-free oil to eliminate background signal and modifications to the image reconstruction hardware to deal with the large quantity of imaging data as well as a custom RF coil. The results shown here suggest that similar data can be readily acquired without taking extraordinary or costly measures.
Postmortem, whole-brain MRI provides an opportunity to investigate anatomy at spatial resolution approaching that of histology without the distortions and limited coverage inherent to histology1,2. Recently, a group presented impressive ex vivo brain images with a dedicated effort for developing an optimized apparatus3. In this abstract, we present a high resolution ex vivo brain image with a relatively simple set up.
Methods
System info : All the images were acquired using a Siemens 7T Magnetom with SC 72 gradient system (Siemens Medical Solutions, Erlangen) with 28ch knee coil (Quality Electrodynamics, Mayfield Village)
Specimen : A brain anonymously donated to the Cleveland Clinic Lerner College of Medicine was used for this study. Although no details are known about the medical history or cause of death of the donor, the brain appears to have been free of significant pathology at the time of death.
Air Bubble Removal: We used a commercial Coleman 1 gallon water jug (model # 3000000739)4 as a container and removed the insulating outer jacket using a band saw to fit the container in the coil (inner diameter 154mm). We sealed the lid spout and other openings with epoxy4. We cut a 120mm diameter HDPE canister into slices to use as spacers to fix the brain position. After filling the container with 10% formalin, we placed the container in a vacuum chamber (LAB1ST, VC30S-8) and reduced the pressure to -28.5 inHg for 5 min to remove bubbles, which distort the images, kept the container under a vacuum of -28.5 inHg for 1 day and let the container sit at atmospheric pressure and room temperature for 5 more days.
Image Parameters :
1. Bubble Check: 2D GRE. TR/TE = 3000/17.8ms , 0.4*0.4*1.5mm, FA 23°, BW 40Hz/pixel, TA= 7min. The long TE enhances sensitivity to air bubbles.
2. Anatomical Imaging: 3D Flash3. TR/TE = 40/14.2ms, 200µm isotropic voxel, FA 25°, BW 90Hz/pixel. Averages = 10. TA = 13.5 hours.
Results
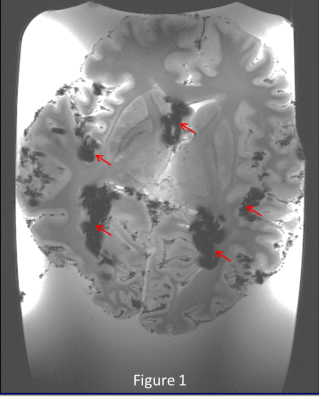
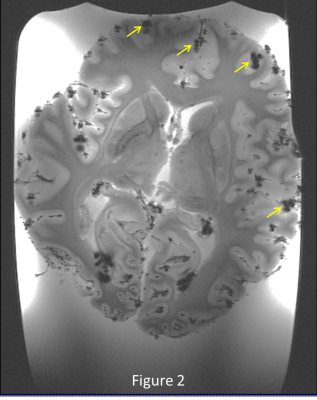
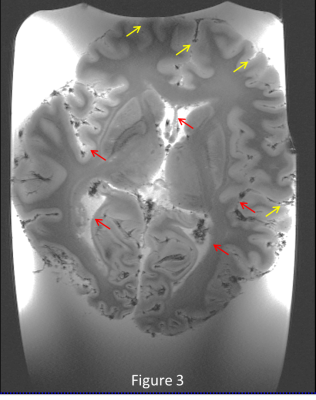
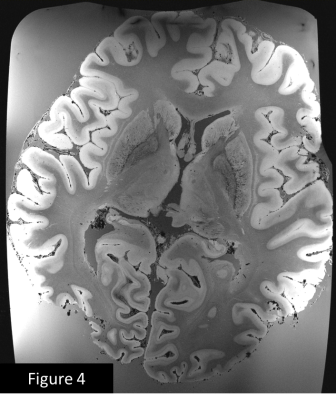
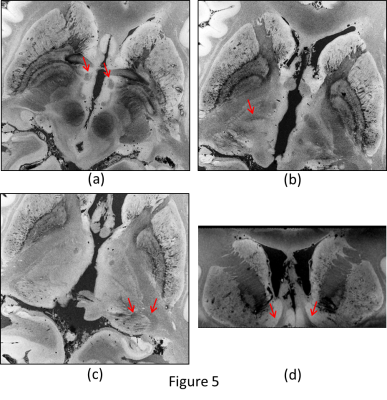
Figure 1 shows the deleterious effect of air bubbles immediately (red arrows) after immersing the brain in liquid. After pumping for 5 minutes and keeping the container under vacuum for 1 day, large bubbles disappear, but many small bubbles (yellow arrows) appear on the outer surface of the brain (figure 2) , but after 5 days of sitting at atmospheric pressure, the small bubbles largely disappear (figure 3). Figures 4 and 5 show 200µm spatial resolution images. Dark areas appear to be arachnoid tissue, not bubbles. In Figure 5, we can clearly see fascicles in the fornices, thalamic nucleui, LGN and adjacent optic radiations and nucleus accumbens. Each of these small structures are of interest for understanding the evolution of neurological disease.
Discussion
Previous work with ex vivo imaging has been done using a sophisticated process with additional steps, such as a custom-built vibration apparatus to remove air bubbles, proton-free oil to eliminate background signal and modifications to the image reconstruction hardware to deal with the large quantity of imaging data3 as well as a custom RF coil. The results shown here suggest that similar data can be readily acquired without taking extraordinary or costly measures. One potential application is to guide histology, which is inherently limited in the spatial extent of tissue that can be interrogated. Of particular interest is the opportunity for exploratory analyses of abnormalities associated with disease that are otherwise occult to the spatial resolution accessible in vivo. Future plans include performing similar studies in diseased brains such as multiple sclerosis, ALS, and Parkinson’s disease.
Acknowledgements
The authors gratefully acknowledge to the anonymous brain donor and Richard Drake, who provided the tissue. We acknowledge technical support provided by Siemens Healthineers.References
1. Augustinack, J. C. et al. Medial temporal cortices in ex vivo magnetic resonance imaging. J Comp Neurol 2013; 521, 4177-4188.
2. Edlow, B. L. et al. Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J Neuropathol Exp Neurol 2012; 71,531-546.
3. Edlow, B. L. et al. 7 Tesla MRI of the ex vivo human brain at 100 micron resolution. Sci Data 6, 244(2019);
4. Shatil, A.S. et al. A Method for Whole Brain Ex vivo Magnetic Resonance Imaging with Minimal Susceptibility Artifacts. Front. Neurol., 2016;7:208
Figures