1755
Intraplatform Repeatability and Interplatform Agreement of Whole-Brain 3D MRE1Radiology, Mayo Clinic, Phoenix, AZ, United States, 2Radiology, Mayo Clinic, Rochester, MN, United States, 3Siemens Medical Solutions USA, Inc., Salt Lake City, UT, United States, 4Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
Using MRE to evaluate the brain requires acquisition of the full 3D wave field and application of 3D inversion processing to generate valid stiffness maps. The goal of this study was to assess the precision and cross-platform reproducibility of whole brain stiffness measurements obtained with prototype 3D SE-EPI-based MRE techniques, as implemented on GE and Siemens MRI platforms, with the goal of standardization. Our data showed excellent intraplatform repeatability and good interplatform agreement.
Introduction
Magnetic resonance elastography (MRE) has widely been employed to measure tissue stiffness noninvasively for assessing liver disease1, 2, and has recently shown promise in evaluating diseases of the brain, such as for the assessment of tumor stiffness3, 4 and adhesion of the tumor to the brain5, 6. These new findings can help the planning of surgical resection and potentially improve patient care. Current commercially available versions of MRE from three major MRI manufacturers employ standardized 2D, scalar (i.e., 1 motion direction) acquisition and data processing techniques that allow quantitative comparisons of liver tissue stiffness across platforms. While a 2D, scalar acquisition and processing provides reliable results in the liver, other applications of MRE, such as evaluating the brain, require the acquisition and inversion processing of a full 3D, vector (i.e., 3 motion directions) wavefield. The goal of this study was to assess the intraplatform repeatability and interplatform agreement of whole-brain 3D MRE on GE and Siemens 3-T MRI scanners.Methods
We developed prototype spin-echo, echo-planar imaging (SE-EPI) MRE sequences with multislice 3D wavefield acquisition and vector motion sensitizing capabilities for Siemens and GE MRI 3-T scanner platforms. Figure 1 shows the major imaging parameters. Ten healthy volunteers without known brain disease underwent a 3-T GE 750W and a 3-T Siemens Skyra scanner with the same flexible brain MRE passive driver. Every subject underwent MRE twice on each scanner, and a total of four times on both scanners. On each scanner, the test-retest performance was assessed to evaluate the intraplatform repeatability by using the intraclass correlation coefficient (ICC). The MRE acquisitions performed on both scanners were used to evaluate the interplatform agreement using the Bland-Altman analysis.Results
Figure 2 shows example MRE magnitude and whole-brain stiffness images acquired twice on the GE and Siemens 3-T scanners. Figure 3 shows the test-retest results for each scanner comparing the whole-brain stiffness, storage modulus and loss modulus. Figure 4 shows the Bland-Altman analysis results comparing the two scanners. Among the ten healthy volunteers, the intraclass correlation analysis showed an excellent intraplatform repeatability: ICC_GE = 0.90, ICC_Siemens = 0.96. The Bland-Altman analysis showed a good interplatform agreement, in general, between the two scaners; the mean paired difference was small. For whole-brain stiffness: mean GE = 2.2 kPa; mean Siemens = 2.0 kPa; mean difference = 0.15 kPa, CI = 0.06 - 0.25 kPa. For storage modulus: GE = 2.0 kPa; Siemens = 1.8 kPa; difference = 0.13 kPa, CI = -0.12 - 0.39 kPa. For loss modulus: GE = 0.6 kPa; Siemens = 0.5 kPa; difference = 0.06 kPa, CI = -0.05 - 0.15 kPa.Discussion and Conclusion
The reported stiffness (and other mechanical properties) of the brain using MRE can be affected by the choice of hardware, software, pulse sequence, scanning parameters and reconstruction methods, which makes cross-study comparisons challenging. Brain MRE was implemented in this study on GE and Siemens 3-T MRI platforms with pulse sequences designed with similar capabilities and parameters to try to minimize these variations, including the use of 2D, multislice, SE-EPI acquisitions; vector motion sensitizing capabilities; and an offline, vendor-independent inversion processing algorithm. Our data showed an excellent intraplatform repeatability and good interplatform agreement.Acknowledgements
No acknowledgement found.References
Venkatesh, S., et al., Magnetic Resonance Elastography of Liver: Technique, Analysis and Clinical Applications, J Magn Reson Imaging. 2013 Mar; 37(3): 544–555.
Kim, D., et al., Comparison of technical failure of MR elastography for measuring liver stiffness between gradient-recalled echo and spin-echo echo-planar imaging: A systematic review and meta-analysis, J Magn Reson Imaging. 2019 Aug 27.
Hughes, J.D., et al., Magnetic resonance elastography detects tumoral consistency in pituitary macroadenomas. Pituitary, 2016. (19)(3): p. 286-92.
Hughes, J.D., et al., Higher-Resolution Magnetic Resonance Elastography in Meningiomas to Determine Intratumoral Consistency. Neurosurgery, 2015. (77)(4): p. 653-8; discussion 658-9.
Yin, Z., et al., Slip Interface Imaging Predicts Tumor-Brain Adhesion in Vestibular Schwannomas. Radiology, 2015. (277)(2): p. 507-17.
Yin, Z., et al., Slip interface imaging based on MR-elastography preoperatively predicts meningioma-brain adhesion. J Magn Reson Imaging, 2017. (46)(4): p. 1007-1016.
Figures
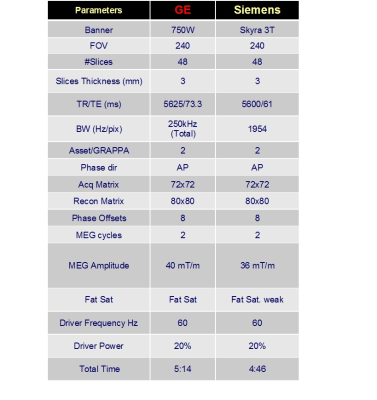
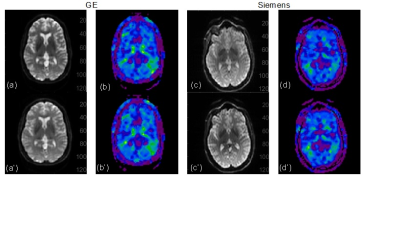
Figure 3. 3D WHOLE BRAIN MRE (a) test-retest on GE 750W; (b) test-retest on Siemens Skyra; (c) Interplatform agreement between both scanners.
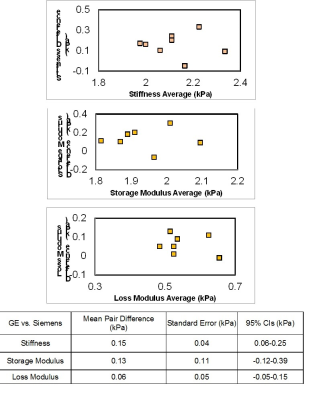
Figure 4. Bland-Altman Analysis of whole-brain 3-D MRE between GE 750W and Siemens Skyra scanners.