1746
Multi-level fiber tracking: evaluation on clinical data
Andrey Zhylka1, Nico Sollmann2,3, Alberto De Luca4, Daniel Krahulec5, Marcel Breeuwer1,5, Alexander Leemans4, and Josien Pluim1
1Biomedical Engineering department, Eindhoven University of Technology, Eindhoven, Netherlands, 2Department of Diagnostic and Interventional Neuroradiology, , Klinikum rechts der Isar, Technische Universität München, Munich, Germany, 3TUM-Neuroimaging Center, Klinikum rechts der Isar, Technische Universität München, Munich, Germany, 4University Medical Center Utrecht, Utrecht, Netherlands, 5MR R&D Clinical Science, Philips Healthcare, Best, Netherlands
1Biomedical Engineering department, Eindhoven University of Technology, Eindhoven, Netherlands, 2Department of Diagnostic and Interventional Neuroradiology, , Klinikum rechts der Isar, Technische Universität München, Munich, Germany, 3TUM-Neuroimaging Center, Klinikum rechts der Isar, Technische Universität München, Munich, Germany, 4University Medical Center Utrecht, Utrecht, Netherlands, 5MR R&D Clinical Science, Philips Healthcare, Best, Netherlands
Synopsis
Conventional deterministic fiber tractography approaches commonly used in clinical applications are prone to generating false-negative reconstructions, which might influence further decision-making related to treatment and repeated surgery in patients with brain tumors. Surgery-related effects, such as blood inflow into white matter and edema, further distort the diffusion signal, complicating the task of tractography. We evaluated a novel multi-level fiber tractography approach on data of subjects who had undergone tumor resection. A comparison with conventional deterministic approaches is performed. The results were correlated with the reported motor-function deficit grades.
Introduction
Diffusion fiber tractography (FT)1 has the potential to become an essential tool for preoperative planning and resection guidance in brain tumor surgery2, mainly by assisting neurosurgeons with delineations of fiber bundles in the vicinity of a tumor3. Diffusion tensor imaging (DTI) based tractography is the dominant technique used in clinical practice4, despite its well-known limitations in the context of FT5. Improving the false-negative rate would allow identification of patients who have intact nerve pathways in functionally eloquent brain areas and could, thus, benefit better from additional treatment in case of a functional deficit. A multi-level fiber tractography (MLFT) algorithm6 was recently proposed to overcome this limitation. We evaluated the MLFT algorithm on brain tumor patients with functional deficits as determined using a standard clinical routine and compared it with conventional deterministic DTI and constrained spherical decomposition (CSD)7 based approaches.Methods
In this work we used follow-up diffusion MRI (dMRI) images of five subjects (Table 1) who had undergone tumor resection, where brain tumor was located in the vicinity of the motor network. Motor deficit grades were provided for patients’ pre-operative and follow-up states according to the muscle strength scale by the British Medical Research Council8 (BMRC) (Table 1).DMRI images were acquired on a 3-Tesla scanner. DMRI contained one volume at b=0s/mm2, 32 volumes at b=1000s/mm2 with uniformly distributed gradient directions. The following preprocessing steps from a clinical neuro-FT application prototype9 were applied to dMRI: global intensity normalization10, brain extraction11, noise removal10, bias field correction10, Gibbs ringing correction10, motion-effect and eddy-current compensation12,13. Additionally, echo-planar imaging distortion correction as well as template-based cortical parcellation were performed with ExploreDTI14.
The MLFT algorithm was used to reconstruct the left and right corticospinal tract (CST) of all subjects from seed regions of interest (ROIs) manually delineated at the top of the brain stem. The motor cortex was chosen as a target ROI based on cortical parcellation. DTI-based and CSD-based whole-brain tractography was run and the pathways were filtered based on ROIs used for MLFT. Radial coverage metric was chosen for the comparison of the algorithms across hemispheres. This metric calculates a sector in coronal motor cortex projection that is populated with fiber tracts.
Results
Given that for patients #1-2 complete function loss was reported subsequent to surgery, they were investigated more closely.Figure 1 shows the reconstruction results for patient #1, with a surgical cavity directly below the left motor cortex. MLFT reconstructs the decompressed left CST. CSD- and DTI-based tractography algorithms were not capable of reconstructing pathways going towards the face motor area.
For patient #1 MLFT (Figure 2) was able to achieve higher radial coverage only in the left hemisphere (LH), constituting a 58-degree sector, while CSD and DTI covered only 26- and 19-degree sectors, respectively. At the same time, all three approaches showed similar performance in the right hemisphere (RH) covering mostly the trunk and leg motor area.
For patient #2 (Figure 3) the results of the MLFT are characterized by a higher radial coverage in both hemispheres, although the sector covered in the right one is smaller. CSD-based tractography reached an increased sector in the LH compared to the RH, where there is a 23-degree sector. However, the left-hemispheric sector contains a gap around the top face motor area. DTI FT covered only the sectors of 30° and 27° in the LH and RH, respectively.
For the remaining patients MLFT primarily outperformed DTI and CSD FT except for the RH of patient #5. The conventional approaches performed comparably (Figure 4).
Discussion
MLFT was able to reconstruct anatomically coherent bundles in most of the cases, suggesting that it can produce satisfactory results in the vicinity of a surgical cavity and delineate decompressed bundles. On the contrary, conventional CSD- and DTI-based tractography algorithms reconstruct mostly the part of the bundle propagating into the superior part of the motor cortex.Underperformance of MLFT on the RH of patient #1 might probably be explained by surgically evoked brain shift, which heavily affected the shape of the CST bundles and, consequently, the diffusion maps.
Complete function loss in the left arm and the left hand was reported at the follow-up stage for both patients. However, in both cases MLFT was able to reconstruct pathways reaching the motor cortex area, which approximately corresponds to the functionality in question. Given that for patient #2 no function loss in arm-hand area was reported pre-operatively and the tumor has no direct effect on the left CST, this patient might potentially benefit from therapy and recover the function. The same might hold for patient #1 regarding the arm-hand function. However, for the leg-foot function for patient #1 no pathways reaching leg motor cortex of the LH were reconstructed by either of the algorithms.
Conclusion
In this work, we have evaluated a novel tractography approach for dMRI of patients with surgically removed brain tumors and compared the performance to conventional deterministic CSD- and DTI-based approaches. The novel MLFT approach produced more extensive coverage of the motor cortex in most of the cases, including pathways that suggest the potential for recovery of function loss. In future, therapy outcome should be added to investigate recovery patterns.Acknowledgements
Andrey Zhylka and Daniel Krahulec are supported by the funding from the European Union's Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No 765148.References
- Jeurissen B., Descoteaux M., Mori S., Leemans A. Diffusion MRI tractography of the brain. NMR Biomed 32(4) (2019) e3785.
- Berman J. Diffusion MR tractogrpahy as a tool for surgery planning. Magn Reson Imag Clinics of North America 17(2) (2009) pp 205-214.
- Javadi S., Nabavi A., Giordano M., Faghihzadeh E., Samii A. Evaluation of diffusion tensor imaging-based tractography of the corticospinal tract. Neurosurgery 80(2) (2016) pp 287-299.
- Farquharson S., Tournier J.-D., Calamante F., Fabinyi G., Schneider-Kolsky M., Jackson G. D., Connelly A. White matter fiber tractography: why we need to move beyond DTI. J of Neurosurgery 118(6) (2013) pp 1367-1377.
- Fortin D., Aubin-Lemay C., Boré A., Girard G., Houde J., Whittingstall K., Descoteaux M. Tractography in the study of the human brain: a neurosurgical perspective. Canad J of Neurosurg Scien 39(6) (2012) pp 747-756.
- Zhylka A., Leemans A., Pluim J., De Luca A. On fiber orientation distribution peak selection for diffusion MRI tractography. Magn Reson Mater Phy (2019) 32(Suppl 1): p 14.
- Jeurissen B., Leemans A., Jones D. K., Tournier J.-D., Sijbers J., Probabilistic fiber tracking using the residual bootstrap with constrained spherical deconvolution, Hum Brain Map 32 (3) (2011) pp 461–479.
- Medical Research Council. Aids to the examination of the peripheral nervous system, Memorandum 45 (1981).
- Krahulec D., Thiele F., Wenzel F., Versluis M., van de Ven K., Breeuwer M. Platform for Enhanced Diffusion MRI Data Processing Pipeline to Guide Tumor Neurosurgery. Magn Reson Mater Phy (2019) 32(Suppl 1): pp 420-421.
- Tournier J.-D., Calamante F., Connelly A., MRtrix: Diffusion tractography in crossing fiber regions, Intern J of Imag Syst and Tech 22 (1) (2012) pp 53–66.
- Garyfallidis E., Brett M., Amirbekian B., Rokem A., van der Walt S., Descoteaux M., Nimmo-Smith I. and Dipy Contributors. DIPY, a library for the analysis of diffusion MRI data. Frontiers in Neuroinf, 8 (2014) p 8.
- M. Jenkinson, C.F. Beckmann, T.E. Behrens, M.W. Woolrich, S.M. Smith. FSL. NeuroImage, 62(2) (2012) pp 782-790.
- Jesper L.R. Andersson and Stamatios N. Sotiropoulos. Non-parametric representation and prediction of single- and multi-shell diffusion-weighted MRI data using Gaussian processes. NeuroImage, 122 (2015) pp 166-176.
- Leemans, A., Jeurissen, B., Sijbers, J., Jones, D. K. ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. ISMRM; 3537 (2009).
Figures

Table 1. Deficit grades of pre-operative and follow-up cases of two subjects
provided for upper extremity (uE) and lower extremity (lE) of both hemispheres
based on BMRC scale. Left and right extremities refer to the left and right
sides of the body.
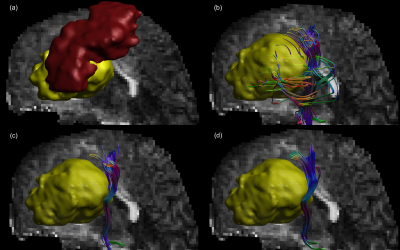
Figure 1. Corticospinal tract reconstruction in case of the cavity (yellow) being
present under the motor cortex (red). Multi-level fiber tractography (b)
reconstructs a compressed bundle maintaining the pathways going to the face area
of the motor cortex. On the other hand, CSD- and DTI-based approaches
reconstruct only the branch going to the arm motor cortex (c and d, respectively)
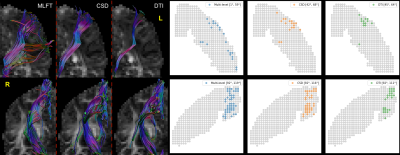
Figure 2. Tractography results for left and right hemispheres of patient #1. The
radial coverage of the motor cortex is improved for the left hemisphere (top
row) when performing multi-level tractography, while other approaches produce
bundles reaching only a narrow motor area. In case of the right hemisphere
(bottom row), the outcome is similar across the approaches.
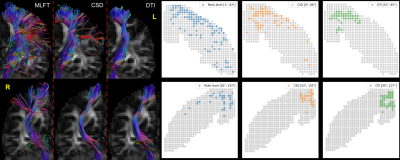
Figure 3. Tractography results for left and right hemispheres of patient #2. Multi-level
tractography improves the motor coverage across hemispheres. CSD-based
tractography was able to reach the lower part of motor cortex in the left
hemisphere (top row), while leaving a gap around the face motor area. For each
of the approaches the reconstruction of the right corticospinal bundle (bottom
row) has decreased angular coverage of the motor cortex than in case of the
left one.
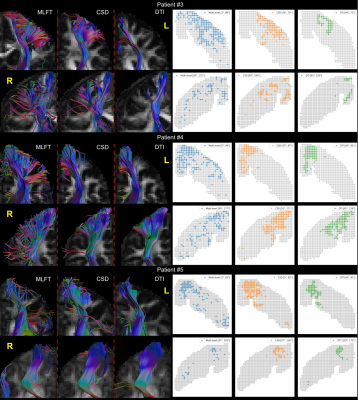
Figure 4. Summary of the tractography results for patients #3-5. In many cases
MLFT outperforms conventional approaches in terms covering the motor-cortex Otherwise,
the performance is comparable.