1739
Do advanced fMRI and tractography preoperative evaluations correspond to intraoperative findings? A pilot evaluation in brain tumour patients.1Neuroradiology, King's College Hospital, London, United Kingdom, 2Neurosurgery, King's College Hospital, London, United Kingdom, 3Computer Assisted Clinical Medicine, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany, 4Department of Biomedical Engineering, School of Biomedical Engineering and Imaging Sciences, London, United Kingdom, 5Department of Medical Physics and Bioengineering, NHS Highland, Inverness, United Kingdom
Synopsis
In this work we have evaluated the feasibility of incorporating advanced fMRI and tractography evaluations into the real-life presurgical management of patients undergoing brain lesion resections. In this pilot cohort of patients, all major preoperative imaging findings were validated by intraoperative measurements. With the inclusion of fMRI end regions, probabilistic tractography allowed a better reconstruction of the corticospinal tract and its branches in regions adjacent or within the tumour with altered or damaged fibre architecture. Robust fMRI-based language lateralization was able to describe likely dominance, in agreement with intraoperative findings and initial postoperative deficit.
Introduction
For the preoperative imaging assessment of brain lesions involving eloquent areas, the use of both language functional MRI (fMRI) and motor fMRI paired with diffusion tensor imaging (DTI) tractography has the potential to inform and assist neurosurgeries1-4. Current presurgical planning systems can support the use of advanced imaging data, but they are almost exclusively restricted to diffusion tensor based tractography, which provides limited depiction of white matter tracts in regions of complex fibre architecture2. FMRI-based language lateralization is also affected by methodological issues: in particular, the conventional single-threshold laterality index is an unreliable and inadequate measure of lateralization5. In this work we evaluate the feasibility of incorporating advanced fMRI and tractography evaluations - which employ probabilistic tractography and robust assessment of language lateralization - into the real-life presurgical management of patients undergoing brain lesion resections. Here we compare preoperative imaging findings with intra and postoperative assessments in a pilot cohort of subjects.Materials and Methods
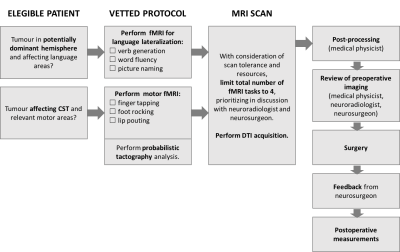
Between April and October 2019, 4 patients (Table in Figure 1) have undergone brain tumour surgery after being offered preoperative imaging evaluations as per flow chart in Figure 2. Head MRI was performed at 1.5T (Siemens Aera, standard 20-channel head-only receive coil). fMRI acquisitions consisted of 6 cycles of alternating rest and activation periods of 30 seconds, and employed a BOLD GRE-EPI sequence (TE/TR=40/3000ms, voxel=2.5x2.5x3mm3). Available tasks were: finger tapping, foot rocking, and lip pouting (motor), and verb generation, word fluency, and picture naming (language lateralization). Activation t-maps were calculated using SPM126. Language laterality was calculated both at hemispherical and regional level using the threshold-independent method proposed by Abbott and colleagues7, and evaluated through comparison against a reference cohort of volunteers8. Diffusion data were acquired using a DTI SE-EPI sequence (TE/TR=86/9500ms, voxel=(2.5mm)3, 6xb=0s/mm2 and 64 diffusion directions at b=1500s/mm2) and processed using constrained spherical deconvolution and probabilistic tractography in MRtrix39. Corticospinal tract (CST) tractography was performed on the hemisphere containing the lesion, and employed the posterior limb of the internal capsule (PLIC) as seed regions, and motor fMRI-based end regions where available. Clinical presurgical brain mapping was performed using Cranial and StealthViz MEDTRONIC Software, and included dissection of the CST with deterministic tractography (available through augmented reality during tumour resection). Intraoperative motor mapping was performed using monopolar probe with a train-of-5 technique (high frequency technique) for both cortical and subcortical areas. Intraoperative language mapping was performed using the Penfield technique (low frequency technique)10. Post resection distances from stimulation were compared with postoperative measurements of CST-resection cavity distance, performed by employing probabilistic tracts (Figure 4d) and considering the distance between the cavity border and the edge of the tract core (5 readings). To allow this, preoperative tractography was superimposed onto postoperative structural images, after applying a transformation matrix estimated from non-linear registration of pre and post surgery T1 volumes11.Results
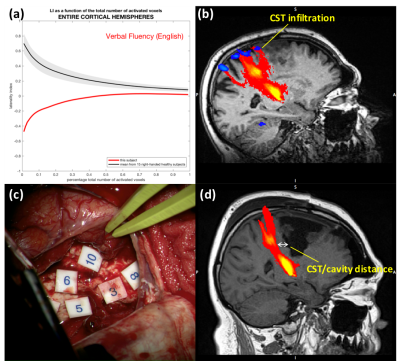
Preoperative and intraoperative findings are summarized and compared in the Table in Figure 3.Language lateralization: this assessment was performed on patients who had the lesion located in the potentially dominant hemisphere (Patient 2-4). Language mapping confirmed predicted hemispherical dominance in all patients (Table in Figure 3). Patient 2 was left handed and right hemisphere dominant according to fMRI (Figure 4a). During supplemental motor area (SMA) resection, the patient experienced spontaneous speech arrest, initiation disturbance, and hesitation, and developed SMA syndrome with speech initiation deficit - only expected in the dominant hemisphere. Patient 3 and 4 (lesion in the non-dominant hemisphere according to fMRI) had no postoperative language deficit.
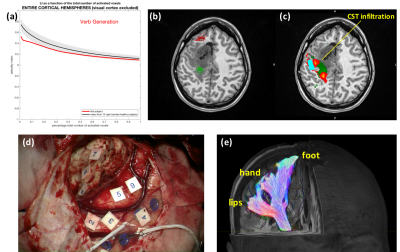
Tractography and motor fMRI: CST was identified in locations predicted by tractography in all patients. Probabilistic tractography also predicted infiltration of the CST (not shown by deterministic tractography) in Patient 2 and 4 (Figure 4b and 5c, respectively), confirmed by intraoperative ultrasound and GLIOLAN imaging. In Patient 4, infiltration of the hand branch might explain preoperative hand movement impairment, and posterior depiction of the foot branch is in keeping with postoperative deficit (Figure 5). Tractography measurements of CST-cavity distances (Table in Figure 3) tended to overestimate the distance measured intraoperatively with stimulation (Figure 4c and 5d), which assumes 1mA~1mm12.
Discussion and Conclusions
In this pilot cohort, all major preoperative imaging findings were validated intraoperatively. With the inclusion of fMRI end regions, probabilistic tractography allowed a better reconstruction of the CST and its branches in regions adjacent or within the tumour with altered or damaged fibre architecture. Robust fMRI-based language lateralization was able to describe likely dominance, in agreement with intraoperative findings and initial postoperative deficit. These advanced evaluations require a constant dialogue and feedback between radiologists, neurosurgeons, and medical physicists involved, to both optimize clinical service resources and to allow cross-validation of MRI and intraoperative findings. Postoperative CST distances are a useful feedback to the neurosurgeon, and are important to understand the relationship between tractography and intraoperative measurements; this relationship, however, might be affected by a number of factors - particularly tract density and geometrical issues (location, orientation, and deformation). Future work will further develop this analysis, validating pre and postoperative tractography CST measurements, and will expand the systematic MRI vs intraoperative findings comparison to a larger cohort.Acknowledgements
This work was carried out at, and supported by, the Department of Neuroradiology at King’s College Hospital NHS Foundation Trust. EDV is supported by the Wellcome/EPSRC Centre for Medical Engineering [WT 203148/Z/16/Z]. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.References
1. Bizzi, A. [2009 ] ‘Presurgical mapping of verbal language in brain tumors with functional MR imaging and MR tractography’. Neuroimaging Clinics 19(4), 573-596.
2. Bucci et al. [2013] ‘Quantifying diffusion MRI tractography of the corticospinal tract in brain tumors with deterministic and probabilistic methods’. NeuroImage: Clinical 3, 361-368.
3. Partovi et al. [2012] ‘Clinical standardized fMRI reveals altered language lateralization in brain tumor patients’. American Journal of Neuroradiology 33(11), 2151-2157.
4. Smits et al. [2007] ‘Incorporating Functional MR Imaging into Diffusion Tensor Tractography in the Preoperative Assessment of the Corticospinal Tract in Patients with Brain Tumors’. AJNR Am J Neuroradiol. 28(7):1354-61.
5. Bradshaw et al. [2017] ‘Methodological considerations in assessment of language lateralisation with fMRI: a systematic review’. PeerJ 5:e3557.
6. Wellcome Trust Centre for Neuroimaging, University College London, UK (www.fil.ion.ucl.ac.uk/spm)
7. Abbott et al. [2010] ‘fMRI assessment of language lateralization: an objective approach’. Neuroimage 50(4):1446-55.
8. Brumer et al. [2019] ‘Relative assessment of language lateralisation with fMRI: evaluation of a novel threshold-independent method’. Proc. Int. Soc. Magn. Reson. Med. 27:3924
9. http://www.mrtrix.org/
10. Szelényi et al. [2010] ‘Intraoperative electrical stimulation in awake craniotomy: methodological aspects of current practice’. Neurosurg Focus 28 (2):E7.
11. Andersson et al. [2007] ‘Non-linear registration aka Spatial normalisation’. FMRIB Technial Report TR07JA2.
12. Nossek et al. [2011] ‘Intraoperative mapping and monitoring of the corticospinal tracts with neurophysiological assessment and 3-dimensional ultrasonography-based navigation. Clinical article’. J Neurosurg. 114(3):738-46.
Figures