1738
Minimizing false streamlines in anatomically constrained tractography for neurosurgery guidance in patients with brain neoplasms
Daniel Krahulec1, Ahmed Radwan2, Stefan Sunaert2, Maarten Versluis1, Kim van de Ven1, and Marcel Breeuwer1,3
1MR R&D Clinical Science, Philips Healthcare, Best, Netherlands, 2Department of Imaging & Pathology, Translational MRI, KU Leuven, Leuven, Belgium, 3Department of Biomedical Engineering – Medical Image Analysis, Eindhoven University of Technology, Eindhoven, Netherlands
1MR R&D Clinical Science, Philips Healthcare, Best, Netherlands, 2Department of Imaging & Pathology, Translational MRI, KU Leuven, Leuven, Belgium, 3Department of Biomedical Engineering – Medical Image Analysis, Eindhoven University of Technology, Eindhoven, Netherlands
Synopsis
A data analysis pipeline comprising KUL neuroimaging tools was applied to analyze diffusion and anatomical MRI datasets with neoplastic lesions and perform diffusion tractography with anatomical priors. We utilized a novel framework for reducing the amount of false positive streamlines to allow for improved visualization of different fiber bundles. Results illustrate the meaningfulness of this approach in neurosurgery workflow through offering clinicians more information on the quality of perilesional fiber bundles as well as where to resect with care to preserve functionally eloquent areas.
Introduction
Brain tumor surgery aims at maximizing the extent of resection while minimizing damage to healthy tissues. Diffusion MRI data help identify nerve fiber bundles, which provide neurosurgeons with important knowledge1, especially in complicated cases with highly aggressive lesions (e.g. glioblastomas). Nowadays, however, this type of information is infrequently used in clinical practice as well as in clinical research applications for several reasons: 1. Commercial software methods for tractography fail to resolve complex nerve fiber architectures (e.g. crossing/fanning/kissing fibers); 2. Use of poor models (e.g. tensor) to find local fiber orientation leads to inaccurate estimates2 of fiber bundle presence in areas of infiltration, displacement or edema; 3. Slow data processing and cumbersome workflow in current clinical practice. Ongoing work (TRABIT design-PhD project3) focuses on solutions to these problems through faster data processing workflow, efficient use of anatomical information and enhanced visualization. (First two researchers in the author list of this abstract share joint first authorship.)Methods
A total of 6 patient datasets, acquired on the Philips Achieva 3T system, were converted from DICOM to NIfTI with compliance to the BIDS4 data structure using KUL neuroimaging tools5. These datasets consisted of 3D T1-weighted (isotropic 0.9mm voxel size), 3D T2-weigted, 3D FLAIR and 4D EPI-dMRI scans (b values: 0, 1200, 2500 with 7, 128 and 128 diffusion directions respectively, and 4 reversed b0 volumes, isotropic 2mm voxel size). Open-source diffusion MRI data analysis tools (MRtrix6, ANTs7) were utilized in the processing pipeline for image artifact corrections (noise, Gibbs ringing, bias field). Both T1-weighted and diffusion images were skull-stripped and coregistered before launching tractography. EPI distortion, eddy-current and motion corrections were performed with FSL8. Segmentation of brain structures was done with Freesurfer9, and lesion masks were derived by manual delineation. For the visualization of tracts and their relationship with lesions, the anatomically constrained tractography framework10 with the constrained spherical deconvolution11 algorithm was applied. Initial fiber tracking used both left and right pre-, para- and postcentral gyri, brain stem and pons as seed regions. Segmented tumor volume was added as an exclusion criterion, and the algorithm was set to stop tracking at an angular fiber deviation of 60°. Tracking terminated at the grey-white matter interface. To minimize the amount of false positive streamlines, a threshold was defined as follows: 1. generate the FD map of the fiber bundle to be filtered; 2. calculate maximum FD value in the image; 3. use 0.3 % (based on empirical testing12) of the max. value to threshold the image; 4. binarize the thresholded image to generate an inclusive mask, which is subsequently used as an inclusion criterion for the tracking algorithm, where fibers passing beyond the mask are rejected. Resulting streamlines were converted to VTK and visualized on underlying T1-weighted images with lesion labels in a VTK-based viewer.Results
In Fig. 1, we demonstrate the lesion infiltration effect on the corticospinal tract and arcuate fasciculus (A); the compression effect on superior longitudinal fasciculus and the inferior fronto-occipital fasciculus (B); the adjacency of optic radiations to tumors (C, D); the destructive effect on the arcuate fasciculus (E); and the compression of the inferior longitudinal fasciculus (F). In Fig. 2, we show a more detailed view of Fig. 1A to highlight the impact of streamline thresholding on two fiber bundles largely affected by the lesion.Discussion & conclusions
Current visualization already provides basic, clinically relevant information on the relationship between lesion mass and adjacent nerve fiber bundles. Applied method allows for a more realistic reconstruction of fiber tracks (Fig. 1), which are known to be highly challenging to the widely used DTI FACT algorithm. The probabilistic nature of fiber tracking used in this work helps reconstruct fiber bundles affected by the pathology. Reconstructed fiber pathways are consistent with anatomical knowledge in healthy subjects, and show evidence of resulting deformation and displacement as expected in diseased cases. The streamline filtering strategy aids in suppressing spurious fiber trajectories, thus providing discrete fiber bundles to be used in specific patient risk estimation in the preoperative stage. Apart from our approach being fully automated, and therefore freed from user bias, it also enables to resolve crossing fibers in periventricular white matter, especially at the centrum semiovale (Fig. 1A).Future work
Future development will focus on further acceleration of the data analysis workflow and its implementation into the Philips IntelliSpace Discovery (ISD) research platform. Using Philips proprietary algorithms, more robust segmentation of brain structures, cortical parcellation13, and tumor segmentation14 (Fig. 3) will be implemented into the data processing workflow. Furthermore, novel visual features, such as simulation of a path to access tumor from head surface, indicating fiber-tracking uncertainty or displaying suggested resection margins, are intended to enhance the visualization of results.Acknowledgements
This research is part of the TRABIT project, which has received funding from the European Union's Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No 765148.References
- Alexopoulos G, Cikla U, El Tecle N, Kulkarni N, Pierson M, Mercier P, et al. The Value of White Matter Tractography by Diffusion Tensor Imaging in Altering a Neurosurgeon's Operative Plan. World Neurosurg. https://doi.org/10.1016/j.wneu.2019.08.168.
- Calamante F. The Seven Deadly Sins of Measuring Brain Structural Connectivity Using Diffusion MRI Streamlines Fibre-Tracking. Diagnostics (Basel). 2019 Sep 6;9(3):E115.
- www.trabit.eu
- bids.neuroimaging.io
- https://github.com/treanus/KUL_NeuroImaging_Tools
- www.mrtrix.org
- stnava.github.io/ANTs/
- fsl.fmrib.ox.ac.uk/fsl/fslwiki/
- https://surfer.nmr.mgh.harvard.edu/
- Smith RE, Tournier JD, Calamante F, Connelly A. Anatomically-constrained tractography: improved diffusion MRI streamlines tractography through effective use of anatomical information. Neuroimage. 2012 Sep;62(3):1924-38.
- Tournier JD, Calamante F, Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. Neuroimage. 2007 May 1;35(4):1459-72.
- Radwan et al. ESMRMB 2019, 36th Annual Scientific Meeting, Rotterdam, NL, Magn Reson Mater Phy 32 (Suppl 1): S235–S371, DOI: 10.1007/s10334-019-00755-1 (2019).
- Wenzel F, Meyer C, Stehle T, Peters J, Siemonsen S, Thaler C, et al. Rapid fully automatic segmentation of subcortical brain structures by shape-constrained surface adaptation. Med Image Anal. 2018 05;46:146-61.
- Perkuhn M, Stavrinou P, Thiele F, Shakirin G, Mohan M, Garmpis D, et al. Clinical Evaluation of a Multiparametric Deep Learning Model for Glioblastoma Segmentation Using Heterogeneous Magnetic Resonance Imaging Data From Clinical Routine. Invest Radiol. 2018 11;53(11):647-54.
Figures
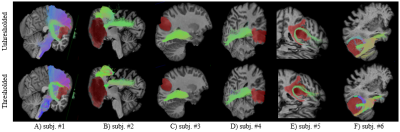
Fig. 1: Fiber tracks with
semitransparent tumor labels in 6 different tumor cases.
Top row: before applying FD threshold. Bottom row: after dropping low FD
voxels. Lesion masks are red-colored.
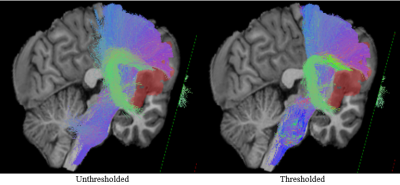
Fig. 2: Zoomed view at the effect of filtering false positive streamlines. The thresholded
representation of the fiber bundles facilitates easier determination of the shape of both the
corticospinal tract and the arcuate fasciculus.
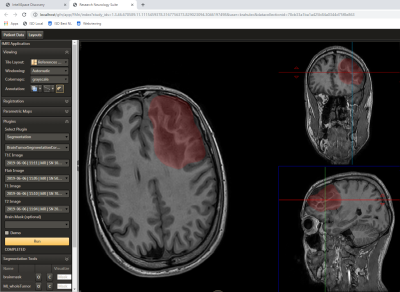
Fig. 3: User interface
of the Philips IntelliSpace Discovery Research Neurology Suite and lesion mask as generated by a Philips-developed
tumor segmentation tool (integrated as an external plugin in the left column).
This setup is being used in current work on the improvement of data processing workflow.