Gao Ankang1, Zhang Huiting2, Yang Guang3, Wang Shaoyu2, Yan Xu2, Bai Jie1, and Cheng Jingliang1
1MRI, Dept. of MRI, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China, 2MR Scientific Marketing, Siemens Healthcare, Shanghai, China, 3Shanghai Key Laboratory of Magnetic Resonance, East China Normal University, Shanghai, China
Synopsis
Diffusion
imaging is widely used to noninvasively detect the
microscopic diffusion properties of biological tissues in vivo. Advanced
diffusion models were recently proposed to provide additional microstructure
information. In present work, we applied four diffusion models in glioma
grading, including DTI, DKI, MAP-MRI and NODDI model, which could be acquired
within a single scan.
Background and Purpose
Diffusion
imaging is widely used to noninvasively detect the
microscopic diffusion properties of biological tissues in vivo. Advanced
diffusion models were recently proposed to provide additional microstructure
information. This study aimed to evaluate the performance 4
diffusion models in glioma grading, including Diffusion tensor imaging (DTI), Diffusion
kurtosis imaging (DKI), Mean apparent propagator (MAP)-magnetic resonance
imaging (MRI), and Neurite orientation dispersion and density imaging (NODDI) models.
A single and comprehensive acquisition scheme was used for all the four models.
Histogram features were extracted from parameters of these diffusion models and
used in grading of glioma.Materials and Methods
Patients
and MRI
The
institutional review board approved this prospective study, and informed
consent was obtained from all patients. Totally 41 patients were recruited,
including 15 low-grade glioma (WHO II, III) and 26
high-grade glioma (WHO IV). All the patients
underwent diffusion weighted imaging (DWI) and conventional MRI examinations on
a 3T MR scanner (MAGNETOM Prisma; Siemens Healthcare, Erlangen, Germany) with a 64 channel of head-neck coil. DWI was
performed using a spin-echo echo-planar imaging sequence and the parameters
were: FOV = 220 × 220 mm2, slice thickness = 2.0 mm,
slices = 66, TR/TE = 3700/72 ms, in-plane acceleration factor = 2,
slice acceleration factor = 2, diffusion time δ/Δ = 15.9/35.0 ms, two b=0 data
and 98 diffusion images with different diffusion gradient directions and bmax=
3000 s/mm2. The DTI, DKI,
MAP, and NODDI parameters were calculated using
an in-house developed post-processing software called NeuDiLab, which is based
on an open-resource tool DIPY (Diffusion Imaging In Python,
http://nipy.org/dipy). The calculated parameters were as follows: fractional
anisotropy (FA), axial diffusivity (AD), radial diffusivity (RD) and mean diffusivity (MD) from DTI and
DKI; axial kurtosis (AK), radial kurtosis (RK) and mean
kurtosis (MK) from DKI; mean squared displacement (MSD), return to the origin
probability (RTOP), return to-the plane probability (RTPP), return to the axis
probability (RTAP), Q-space Inverse Variance (QIV), perpendicular
non-Gaussianity (NG⊥),
parallel non-Gaussianity (NG//) and non-Gaussianity (NG) from
MAP-MRI; intracellular volume fraction (ICVF), orientation dispersion index
(ODI) and isotropic
volume fraction (ISOVF) from NODDI.
The ROI drawn
The region-of-interest (ROI) was
manually drawn around the entire tumor on axial T2-weighted images or axial post-contrast
3D T1-weighted magnetization prepared rapid acquisition with gradient echo
(MPRAGE) images. Then the
ROIs were copy to all the calculated diffusion maps. Then, the histogram
features of all parameters were automatically extracted from their
corresponding ROIs.
Histogram
Analysis
A statistical model based
on histogram features was constructed to predict glioma grading using FeAture
Explorer software (FAE, v0.1.1, https://github.com/salan668/FAE). The main
technological processes were as follows. First, the normalization and clean of
feature was performance. In order to balance the two sets of samples, the data
of negative group was up-sampled. 41 cases were selected as the training and validation
data set. Then, the dimension of the feature space was reduced. If the cosine
value of the feature pair was larger than 0.86, one of them was randomly removed.
Before model development, recursive feature elimination (RFE) was used to
select features. Logistic regression was used as the classifier. A hyper-plane
was searched in the high dimension to separate the samples. To prove the
performance of the model, cross validation with 10-folder on the data set was
applied. Finally, The performance of the model was evaluated using receiver
operating characteristic (ROC) curve analysis and the area under the ROC curve
(AUC). The accuracy, sensitivity, specificity, positive predictive value (PPV),
and negative predictive value (NPV) were also calculated at a cutoff value equivalent
to the maximum value of the Yorden index.Results
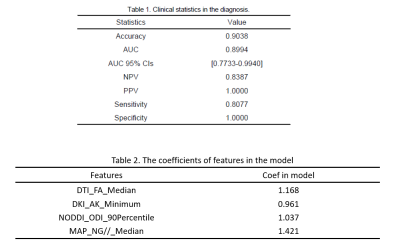
The clinical statistics and
the selected features in the prediction model were shown in Table 1 and Table
2. Finally, four features, including the
median of FA from DTI, the minimum value of AK, the 90 percentile of ODI, and
the median of NG//, were
used in the model to achieve AUC and accuracy at 0.899 and 0.904, respectively,
which is the highest AUC on the validation data set. The ROC curve was shown in
Figure 1.Discussion
As
reported in previous studies [1-4], MD and MK were more useful in
glioma grading. In this study, four diffusion models and histogram parameters
were analysis here, and the results found that the combination of these models
could generate the best overall prediction power for glioma grading. The
optimized features combination are generated from four models (AK, FA, ODI, and
NG// are from DKI, DTI, NODDI
and MAP-MRI). The good performance of this combination may be due to the
multiple dimension of the microstructure information these models offered.Conclusion
Histogram Analysis based on multiple diffusion models showed a great
potential in glioma grading.Acknowledgements
No acknowledgement found.References
[1] Qi Xi-Xun, Shi Da-Fa, Ren Si-Xie, et
al. Histogram analysis of diffusion kurtosis imaging derived maps may
distinguish between low and high grade gliomas before surgery.[J]. European
radiology,2018,28(4).
[2] Hempel Johann-Martin, Brendle Cornelia,
Bender Benjamin, et al. Diffusion kurtosis imaging histogram parameter metrics
predicting survival in integrated molecular subtypes of diffuse glioma: An
observational cohort study.[J]. Europe-an journal of radiology,2019,112.
[3]
A. Vamvakas,S.C. Williams,K. Theodorou, et al. Imaging biomarker analysis of
advanced multiparametric MRI for glioma grading[J]. Physica Medica,2019,60.
[4] Raja Rajikha, Sinha Neelam, Saini Jitender, et al. Assessment
of tissue heterogeneity using diffusion tensor and diffusion kurtosis imaging
for grading gliomas. [J]. Neuroradiology,2016,58(12).