1732
Diffusion Histology Imaging Characterizes and Differentiates Various Tumor Histological Features in High-Grade Brain Tumors1Radiology, Washington University School of Medicine, St. Louis, MO, United States, 2Medical Scientist Training Program, The University of Alabama at Birmingham, Birmingham, AL, United States, 3School of Medicine, University of Missouri - Kansas City, Kansas City, MO, United States, 4Biomedical Engineering, Washington University, St. Louis, MO, United States, 5Keck School of Medicine, The University of Southern California, Los Angeles, CA, United States, 6Pediatrics, Washington University School of Medicine, St. Louis, MO, United States, 7Pathology and Immunology, Washington University School of Medicine, St. Louis, MO, United States
Synopsis
Current clinical diagnosis, surgical resection, and assessment of treatment response for high-grade brain tumor patients relies heavily on gadolinium-enhanced T1-weighted MRI, although such imaging is non-specific for tumor and merely reflects a disrupted blood-brain barrier. The complex tumor microenvironment and spatial heterogeneity make high-grade brain tumor very difficult to characterize using current clinical imaging modalities. We developed a novel imaging strategy to characterize key tumor histological features and demonstrated its capability to accurately predict these histology. Extending this approach to larger cohorts of both tumor specimens and patients could provide further validation and facilitate its clinical translation for patient management.
Introduction
Brain tumors cause substantial cancer-related mortality and morbidity and are the leading cause of cancer-related death in children in the U.S.1 Current clinical guidelines recommend use of gadolinium-enhanced T1-weighted imaging to assess high-grade brain tumors.2, 3 However, despite the use of multiparametric imaging (mpMRI) modalities, diagnoses often fail to accurately reflect tumor histopathology, namely cellular density, necrosis, hemorrhage, and infiltrated edges.We previously developed diffusion basis spectrum imaging (DBSI)4 and demonstrated its ability to quantitatively characterize pathologies in multiple central nervous system diseases, including multiple sclerosis,5 spinal cord injury6 and epilepsy.7 We modified DBSI to better characterize these pathology-directed structural changes to ultimately apply DBSI-derived diffusion metrics along with a deep artificial neural network (DNN) algorithm in detecting and differentiating various tumor histological features in high-grade brain tumors. Here we name this imaging strategy as diffusion histology imaging (DHI).
Methods
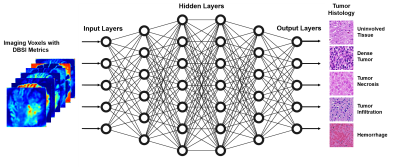
Thirty-one brain tumor specimens from six autopsied pediatric and adult brains including embryonal neoplasms and diffuse high-grade gliomas were studied. Specimens were formalin-fixed at time of collection and were scanned using a 4.7T MR scanner (Agilent Technologies, Santa Clara, CA) with a home-made surface coil (4-cm diameter). A multi-echo spin-echo diffusion-weighted sequence with 99 diffusion-encoding directions and maximum b-values of 3000 s/mm2 was employed to acquire diffusion-weighted images with 0.25×0.25 mm2 in-plane resolution and 0.5-mm thickness. DBSI analysis was performed on each image voxel using an in-house developed MATLAB (Mathworks, Natik, MA). Representative sections were submitted for routine histologic processing and H&E staining.A supervised deep-neural-network (DNN) was adopted to detect and predict histopathologic features by referencing H&E findings. DNN models were developed using TensorFlow 2.0 framework in Python.8 Generally, DNN model was equipped with ten fully-connected hidden layers as well as batch normalization and dropout layers for model optimization and regularization (Fig. 3). The final layer was a fully connected softmax layer that produced a likelihood distribution over five output classes. Adam optimizer was used with default parameters of β1=0.9 and β2=0.999 and a mini-batch size of 100. The hyper-parameters of network architecture and optimization algorithm were chosen through a combination of grid search and manual tuning. Confusion matrices were used to illustrate the tumor histology where DNN prediction contradicted neurologist’s diagnoses. One-versus-rest classification strategy was used to perform ROC and precision-recall (PR) analysis on DNN model’s ability to discern specific histology. Area under curve (AUC) values were calculated to test overall performance.
Results
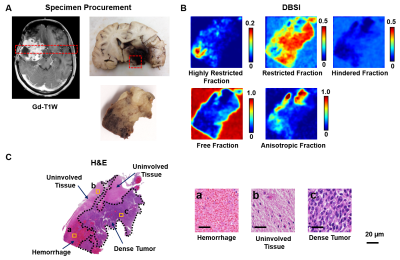
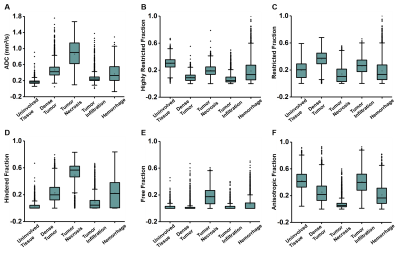
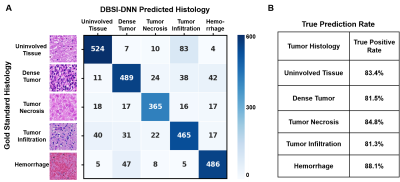
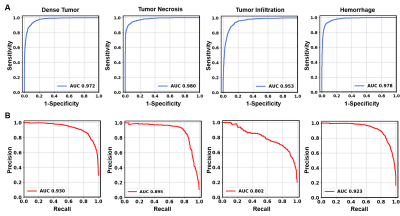
Figure 1 showed a representative case from a 16-year-old pediatric brain tumor patient with embryonal neoplasm (WHO Grade IV). Gd-enhanced T1-weighted image (in vivo) indicated a large lesion in the right posterior region (Fig. 1A). Autopsy specimen showed a mass in the area of the right thalamus, indicating large scale hemorrhage mixed with tumor and necrosis. One tissue block was obtained from this region for analysis. Contrast maps of DBSI-derived diffusion metrics demonstrated hyperintense in areas of highly restricted fraction, restricted fraction and anisotropic fraction. These findings aligned well with areas of hemorrhage, dense tumor, and uninvolved brain parenchyma shown in the corresponding H&E image (Fig. 1C). MR images were co-registered with H&E images, and a total of 57,389 imaging-voxels from segmentations of the five different tumor histologies were obtained and plotted for group comparison (Fig. 2). As expected, tumor necrosis showed higher values on ADC, hindered fraction and free fraction than other histology. Dense tumor and tumor infiltration showed higher restricted fraction values than other histology. Uninvolved tissue and tumor infiltration had higher anisotropic fraction values than other histology.After data balancing, a total of 22,326 imaging voxels were used to train DHI with 5,582 remaining voxels equally split for validation and test sets. For the independent test set (n=2,791), confusion matrix indicated strong overall concordance between DHI predictions and neuropathologist-confirmed histology (Fig. 4A). For this multi-classification, DHI accurately predicted tumor histology—namely dense tumor, tumor necrosis, tumor infiltration, hemorrhage, and uninvolved tissue, with true prediction rates of 83.4%, 81.5%, 84.8%, 81.3% and 88.1%, respectively. We also adopted one-versus-rest strategy to perform ROC and precision-recall analysis to test the model’s ability to differentiate individual tumor histology. DHI demonstrated strong sensitivity and specificity with all five histologies (Fig. 5A, all ROC-AUC>0.95). As ROC analysis is insensitive to class imbalance and could overestimate model performance, we included PR curves to provide complementary information. As expected, PR-AUC values were lower for tumor infiltration detection (0.802) when compared to dense tumor (0.930), necrosis (0.895) and hemorrhage (0.923), respectively.
Discussion and Conclusion
DBSI-diffusion-metrics correlated with certain brain tumor histology. By incorporating a DNN algorithm, we were able to evaluate most components of high-grade brain tumors with an overall accuracy of 83.8%. The predictive performance of DHI on tumor infiltrating edges was not as good, which could be due to the highly variable degrees of infiltration with different tumor cellularity. For example, infiltrative edges with mild to intermediate tumor cellularity could be misinterpreted to be uninvolved tissue. While precise prediction of infiltrating edges remains a limitation of this technique, the collective findings are encouraging because if validated in larger studies, these data indicate the potential of DHI and DBSI to aid targeted biopsies in different brain regions, with the goal of improved diagnostic and prognostic yields.Acknowledgements
This work was supported in part by NIH R01-NS047592, P01-NS059560, U01-EY025500, National Multiple Sclerosis Society (NMSS) RG 5258-A-5, RG 1701-26617, and Department of Defense Idea Award W81XWH-12-1-0457, and The Taylor Rozier Hope for a Cure Foundation.References
1. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011-2015. Neuro-oncology. 2018 Oct 1;20(suppl_4):iv1-iv86.
2. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010 Apr 10;28(11):1963-72.
3. Macdonald DR, Cascino TL, Schold SC, Jr., Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1990 Jul;8(7):1277-80.
4. Wang Y, Wang Q, Haldar JP, et al. Quantification of increased cellularity during inflammatory demyelination. Brain : a journal of neurology. 2011 Dec;134(Pt 12):3590-601.
5. Wang Y, Sun P, Wang Q, et al. Differentiation and quantification of inflammation, demyelination and axon injury or loss in multiple sclerosis. Brain : a journal of neurology. 2015 May;138(Pt 5):1223-38. 6. Sun P, Murphy RKJ, Gamble P, George A, Song SK, Ray WZ. Diffusion Assessment of Cortical Changes, Induced by Traumatic Spinal Cord Injury. Brain Sci. 2017 Feb;7(2).
7. Zhan J, Lin TH, Libbey JE, et al. Diffusion Basis Spectrum and Diffusion Tensor Imaging Detect Hippocampal Inflammation and Dendritic Injury in a Virus-Induced Mouse Model of Epilepsy. Frontiers in neuroscience. 2018 Feb 15;12.
8. Mart, #237, Abadi n, et al. TensorFlow: a system for large-scale machine learning. Proceedings of the 12th USENIX conference on Operating Systems Design and Implementation; Savannah, GA, USA. 3026899: USENIX Association; 2016. p. 265-83.
Figures