1720
A radiomics model for preoperative prediction of brain invasion in meningioma based on MRI1Radiology, Lanzhou University Second Hospital, Lanzhou, China, 2School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, Shanghai, China, 3CAS Key Laboratory of Molecular Imaging, Institute of Automation, Beijing, Beijing, China, 4Lanzhou University Second Hospital, Lanzhou, China
Synopsis
Objectives Using a radiomics method to predict brain invasion by meningioma. Methods 1595 quantitative imaging features were extracted. LASSO was performed to select features. SVM was used to fit the predictive model. Furthermore, a nomogram was constructed, and validated using decision curve analysis (DCA). Results 8 features were significantly correlated with brain invasion. The radiomics model derived from the fusing MRI sequences resulted in the best discrimination ability, with AUC of0.855(95%CI, 0.829-0.882), sensitivity of 80.32% (95%CI, 75.56%-85.25%). Conclusions The radiomics model developed in this study provided a new non-invasive way to facilitate the preoperative prediction of brain invasion in meningioma.
Introduction
Meningiomas are the most common primary intracranial tumors in adults, accounting for 36.7% of all intracranial tumors1. In the latest 2016 edition of the World Health Organization Classification of Central Nervous System tumors2, microscopic examination of brain invasion has been added as an independent grading criterion for the diagnosis WHO grade II atypical meningioma. Brain invasion in neuropathological analyses has gained distinct change in clinical behavior, which was a risk factor of preoperative seizures and postoperative hemorrhage3,4, directly impacting histopathologic grading and therefore eventually therapeutic decision3,5,6. So preoperative prediction of brain invasion is very important. Histopathological examination is the only gold standard for the diagnosis of brain invasion recommended by the guide. However, some studies reported that the frequency of brain invasion was significantly different from neuropathological tissue samples3,5.Since the tumour with the adjacent brain does not allow definite conclusion of brain invasion, and with the absence of neural tissue, exact neuropathologic evaluation of brain invasive is hardly possible, a quite portion of invasive tissue might not be detected during microscopical analyses. Some grade I meningiomas (14.76% in our study) had brain invasion, which would affect the treatment selection and prognosis of patients when pathological examination was missed. Compared to focusing on local fine structures, imaging examinations of brain invasion analyse the whole focus without invading brain tissue. But current image-guided brain invasion testing is non-specific and highly subjective 3,1, depending on the experience of radiologists. To overcome all these problems, radiomics analysis is a reasonable tool which cannot be observed by naked eyes, such as texture, intensity and shape features7,8.Thus ,In this study, we developed and validated a radiomics model to show the potential association between radiomic signatures and brain invasion.Methods
A total of 1070 patients with histopathologic diagnosis of meningioma(including WHOI grade,II grade and III grade) who received MRI preoperative examinations were enrolled. All the tumors were segmented based on CE-T1 and T2, from which 1595 quantitative imaging features were extracted by the pyradiomics. Least absolute shrinkage and selection operator (LASSO) was performed in order to select the most informative features. Subsequently, a linear support vector machine (SVM) was used to fit the predictive model. Furthermore, a nomogram was constructed by incorporating clinical risk factors and radiomics signature, and the clinical usefulness of the nomogram was validated using decision curve analysis (DCA).Results
From 3190 radiomic features (1595 features from CE-T1WI, 1595 features from T2WI), 8 CE-T1WI features and 8 T2WI features were significantly correlated with brain invasion. During all the clinical factors, only the gender information was statistically relevant with brain invasion. The radiomics model derived from the fusing MRI sequences resulted in the best discrimination ability for risk prediction of brain invasion, with AUC of 0.855 (95% CI, 0.829-0.882), sensitivity of 80.32% (95% CI, 75.56%-85.25%).Discussion
With release of the 2016 edition of the WHO Classification of CNS Tumours9,brain invasion in meningiomas has gained highest clinical relevance which directly impacts grading , and therefore influences patients’ prognosis, adjuvant therapy and clinical trials10. Currently ,brain invasion can only be determined by histopathological examination 4,11,but frequencies of brain invasion is closely related to tissue samples3,11,and a considerable part of invasive tissue may not be detected3. A better way was needed for clinicians to discriminate brain invasion before surgery, in order to clinical decision making and patients communication, especially if the tumor is located in the functional area. In our retrospective study, we investigated radiomic analysis based on preoperative MRI to predict brain invasion in patients with meningioma which is the first study. A multi-sequence (combiningCET1WI and T2WI) model of radiomic sequences demonstrated the best predictive power (AUC: 0.855). Meantime, the nomogram ,based on radiomics features and clinical factors, was developed and evaluated to predict brain invasion in meningiomas. The results showed the best performance of prediction of brain invasion that is non-invasive and convenient. In this study, eight features were selected from the CE T1WI and T2WI after feature selection, respectively .Among these features, most were textural features of images, which demonstrated microscopic description of the tumour including cellularity, degenerative changes, peritumoral edema,and destruction and compression of normal brain tissue by tumour, some of cannot be easily identified by humans visual system1,12,13. Moreover, we selected CE T1WI and T2WI sequences to develop model, because CET1WI are often used to determine the boundaries of gross tumours , to reflect the blood supply of tumour and to assess the extent of tumours’ invasion14, especially most meningiomas are rich in blood. T2WI sequences are sensitive to water tissue and can be used to detect the presence of edema and to estimate cellular density15.Therefore , we analyzed CET1WI and T2WI together, a multi-sequence (combining CET1WI and T2WI) model of radiomics features demonstrated the better predictive power than CET1WI or T2WI model respectively.Conclusions
The clinicoradiomic model incorporating the fusing radiomic features and gender information showed good performance for risk prediction of brain invasion. The radiomics model developed in this study provided a new non-invasive way to facilitate the preoperative prediction of brain invasion in meningioma, which could only be achieved based on histopathology in the past.Acknowledgements
We greatly thank Dr. Kuan Yao at School of Biomedical Engineering, Shanghai Jiao Tong University, for technical assistance of laboratory works.References
1.Yae Won Park,JongminOh,Seng Chan You,et al. Radiomics and machine learning may accurately predict the grade and histological subtype in meningiomas using conventional and diffusion tensor imaging. European Radiology.2018; https://doi.org/10.1007/s00330-018-5830-3
2. Louis DN,Perry A,Reifenberger G,et al.The 20 l 6 World Heath Organization Classification of Tumours of the Central Nervous System:a summary.Acta Neuropath01.2016;13l:803—820
3.Alborz Adeli, Katharina Hess, Christian Mawrin,et al. Prediction of brain invasion in patients with meningiomas using preoperative magnetic resonance imaging.Oncotarget. 2018; 9 (89): 35974-35982
4. Chernov M. Letter to the Editor: seizures and invasive meningiomas. J Neurosurg. 2016; 125:1615–16. https://doi.org/10.3171/2016.8.JNS161962
5.Heß K, Spille DC, Wagner A, et al. Letter: brain invasion in meningiomas-sex-associated differences are not related to estrogen and progesterone receptor expression. Neurosurgery. 2017; 81:E25–27.https://doi.org/10.1093/neuros/nyx147.
6.Goldbrunner R, Minniti G, Preusser M, et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016; 17:e383– 91. https://doi.org/10.1016/S1470-2045(16)30321-7.
7.Lambin P, Rios-Velazquez E, Leijenaar R, et al.Radiomics:extracting more information from medical images using advanced feature analysis. Eur J Cancer.2012;48:441–446
8.Aerts HJ, Velazquez ER, Leijenaar RT et al. Decoding tumour phenotype by noninvasive imaging using a quantitative adiomics approach. Nat Commun.2014;5:4006
9.Perry A, Louis DN, von Deimling A, et al. WHO Classification of Tumors of the Central Nervous System. Lyon: International Agency on Cancer Research. 2016:232-245.
10.Jenkinson MD, Javadpour M, Haylock BJ, et al. The ROAM/EORTC-1308 trial: Radiation versus Observation following surgical resection of Atypical Meningioma: study protocol for a randomised controlled trial. Trials. 2015; 16: 519.
11.Brokinkel B, Hess K, Mawrin C. Brain invasion in meningiomas-clinical considerations and impact of neuropathological evaluation: a systematic review. Neuro-oncol. 2017; 19:1298–307. https://doi.org/10.1093/neuonc/nox071.
12.Yang Zhang, Jeon-Hor Chen, Tai-Yuan Chen,et al. Radiomics approach for prediction of recurrence in skull base meningiomas, Neuroradiology,https://doi.org/10.1007/s00234-019-02259-0
13Yan PF, Yan L, Hu TT, et al. The potential value of preoperative MRI texture and shape analysis in grading meningiomas: a preliminary investigation. Transl Oncol.2017;10(4):570–577
14 Zhou M, Scott J, Chaudhury B, et al. Radiomics in brain tumor: image assessment, quantitative feature descriptors, and machine-learning approaches. AJNR Am J Neuroradiol 2018;39:208–216.
15 Qiuyu Wang, Qingneng Li, Rui Mi, Hai Ye, Heye Zhang,Baodong Chen, er al. Radiomics Nomogram Building From Multiparametric MRI to Predict Grade in Patients With Glioma: A Cohort Study. Journal of Magnetic Resonance Imaging, 2018;DOI: 10.1002/jmri.26265
Figures
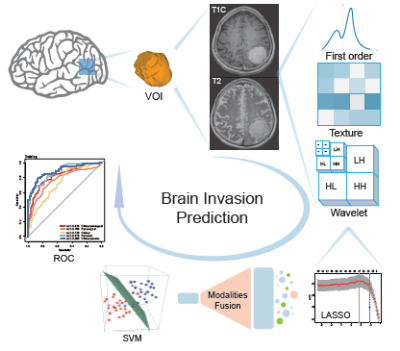
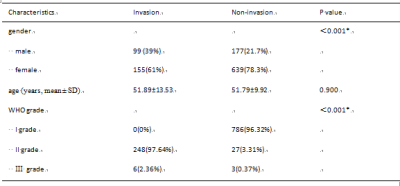
Table 1 Clinical characteristics of patients.
Note: The chi-square test was used to compare the difference in gender and WHO grade, while a Student's t-test was used to compare the difference in age.*P <.05. SD, standard deviation.

Table 2 Performance of sequences models.
Note: CET1WI, contrast-enhanced T1-weighted imaging; T2WI, T2-weighted imaging; ACC, balanced accuracy; AUC, area under receiver operating characteristic curve; SEN, sensitivity; SPE, specificity; PPV, positive predictive value; NPV, negative predictive value