1712
Increased cerebral blood volume in cortical and subcortical brain regions in Glioblastoma patients after EPI distortion correction1Department of Diagnostic Physics, Oslo University Hospital, Oslo, Norway, 2The Intervention Centre, Oslo University Hospital, Oslo, Norway, 3Department of Neurosurgery, Oslo University Hospital, Oslo, Norway, 4Department of Neurosurgery, Geneva University Hospitals, Geneva, Switzerland
Synopsis
We studied the changes in rCBV
introduced by echo-planar imaging (EPI) distortion correction methods when
applied to EPI dynamic susceptibility contrast (DSC)-MRI. The results obtained
using FSL TOPUP and EPIC distortion correction methods indicate that both
subcortical and cortical regions are affected and returning an overall increase
in rCBV. In the context of longitudinal EPI-based analysis in glioblastoma
patients, EPI distortions and subsequent corrections are important determiners for
assessing robust responses of rCBV change.
Introduction
Dynamic Susceptibility Contrast (DSC)-based cerebral blood volume (CBV) is a useful imaging biomarker in glioblastoma patients and it has proven valuable in both predicting overall survival and treatment response1,2. CBV is typically derived from multiple Echo-planar Images (EPIs) during injection of an intravascular tracer3. EPI-based acquisition methods are inherently limited by geometric distortions4,5 that can be corrected using different distortion correction algorithms. To investigate the impact on CBV change from EPI distortion correction, we evaluated two correction methods, FSL TOPUP4 and EPIC5, on pre-treatment DSC-data from 45 patients with glioblastoma. We here present brain regions where rCBV is prone to change from EPI corrections. To relate the regions to tumor location, we count the number of patients with enhancing, necrotic and edema tumor overlapping those regions.Methods
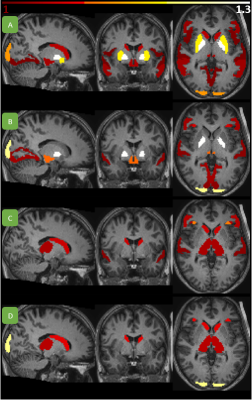
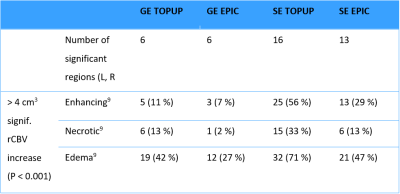
First, using TOPUP and EPIC, we obtained uncorrected and corrected rCBV maps from 45 spin-echo and gradient-echo EPIs in nordicICE (NordicNeuroLab, Bergen, Norway)6. The rCBV maps were normalized to normal-appearing reference tissue, as well as corrected for contrast agent leakage using the Weisskoff method7. Second, the rCBV maps were resliced to MNI space in SPM12 using 3D T2-FLAIR images from the same MRI exam as basis. Third, total rCBV before and after EPI correction were assessed in 66 brain regions in NMI space8. Tumors, ventricles and cerebrospinal fluid were excluded from the analysis and symmetric left and right brain regions were merged for simplicity. Fourth, apparent changes in total rCBV were determined using two-sided paired Wilcoxon signed rank tests with Bonferroni correction for multiple comparisons. Finally, we summarized the number of patients to have at least 4cm3 of enhancing, necrotic and edema tumor overlapping with brain regions depicting a significant change in rCBV following EPI correction.Results
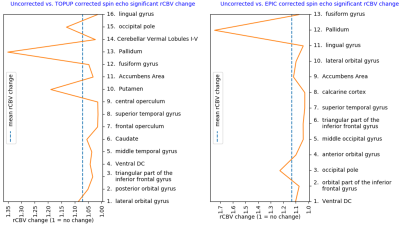
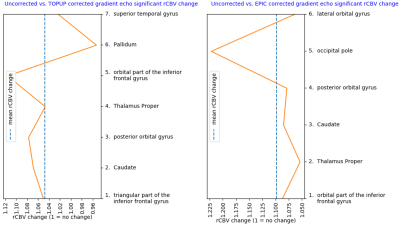
Of the regions with significant (P < 0.001) change in rCBV following distortion correction, all but one region depicted an increase in CBV (Figure 1 and 2). Regions with CBV increase included the pallidum, putamen, occipital pole and caudate nucleus. EPIC corrected spin-echo EPIs had the highest mean increase in rCBV (~13%), whereas TOPUP corrected spin-echo EPIs had a higher number of regions with high rCBV increase (4 of 16 regions above mean increase) (Figures 1-3). Moreover, correction of spin-echo EPIs led to a higher number of regions with significant rCBV change compared to gradient-echo EPIs. Of note, 56% of the patients had at least 4cm3 of enhancing tumor overlapping with significantly increasing rCBV from TOPUP corrected spin-echo EPIs (Table 1).Discussion
The EPI corrections returned a shift towards higher rCBV values in subcortical and cortical areas. Consequentially, our results indicate that if EPI distortions are not corrected, the rCBV values in these regions could be underestimated. Because the apparent magnetic susceptibility as observed in the tissue may also change with the natural history of the disease and treatment, subsequent uncorrected estimates of parameters like rCBV may also reflect unwanted EPI distortions.Conclusion
Our results indicate that EPI distortion correction tends to increase total rCBV in subcortical and cortical areas when analyzed in MNI space. Moreover, because various degrees of EPI distortions may occur during treatment of brain cancer, the estimation of CBV values will likely be affected if not corrected for.Acknowledgements
References
1Schmainda, K. M., Zhang, Z., Prah, M., Snyder, B. S., Gilbert, M. R., Sorensen, A. G., … Boxerman, J. L. (2015). Dynamic susceptibility contrast MRI measures of relative cerebral blood volume as a prognostic marker for overall survival in recurrent glioblastoma: Results from the ACRIN 6677/RTOG 0625 multicenter trial. Neuro-Oncology, 17(8), 1148–1156. https://doi.org/10.1093/neuonc/nou364
2Kong, D.-S., Kim, S. T., Kim, E.-H., Lim, D. H., Kim, W. S., Suh, Y.-L., … Nam, D.-H. (2011). Diagnostic Dilemma of Pseudoprogression in the Treatment of Newly Diagnosed Glioblastomas: The Role of Assessing Relative Cerebral Blood Flow Volume and Oxygen-6-Methylguanine-DNA Methyltransferase Promoter Methylation Status. American Journal of Neuroradiology, 32(2), 382–387. https://doi.org/10.3174/ajnr.A2286
3Østergaard, L., Weisskoff, R. M., Chesler, D. A., Gyldensted, C., & Rosen, B. R. (1996). High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magnetic Resonance in Medicine, 36(5), 715–725. https://doi.org/10.1002/mrm.1910360510
4Andersson, J. L. R., Skare, S., & Ashburner, J. (2003). How to correct susceptibility distortions in spin-echo echo-planar images: Application to diffusion tensor imaging. NeuroImage, 20(2), 870–888. https://doi.org/10.1016/S1053-8119(03)00336-7
5Holland, D., Kuperman, J. M., & Dale, A. M. (2010). Efficient Correction of Inhomogeneous Static Magnetic Field-Induced Distortion in Echo Planar Imaging. NeuroImage, 50(1), 175. https://doi.org/10.1016/j.neuroimage.2009.11.044
6Bjørnerud, A., & Emblem, K. E. (2010). A Fully Automated Method for Quantitative Cerebral Hemodynamic Analysis Using DSC–MRI. Journal of Cerebral Blood Flow & Metabolism, 30(5), 1066–1078. https://doi.org/10.1038/jcbfm.2010.4
7Boxerman, J. L., Schmainda, K. M., & Weisskoff, R. M. (2006). Relative Cerebral Blood Volume Maps Corrected for Contrast Agent Extravasation Significantly Correlate with Glioma Tumor Grade, Whereas Uncorrected Maps Do Not. American Journal of Neuroradiology, 27(4), 859–867. Retrieved from http://www.ajnr.org/content/27/4/859
8Landman, B., & Warfield, S. (2012). MICCAI 2012 workshop on multi-atlas labeling. Medical Image Computing and Computer Assisted Intervention Conference.
9Juan-Albarracín, J., Fuster-Garcia, E., García-Ferrando, G. A., & García-Gómez, J. M. (2019). ONCOhabitats: A system for glioblastoma heterogeneity assessment through MRI. International Journal of Medical Informatics, 128, 53–61. https://doi.org/10.1016/j.ijmedinf.2019.05.002
Figures