1710
Time-efficient method for detecting subtle blood-brain barrier leakage – a clinical feasibility study1Department of Biomedical Engineering, Eindhoven University of Technology, Eindhoven, Netherlands, 2Department of Radiology and Nuclear Medicine, Maastricht University Medical Center, Maastricht, Netherlands, 3School for Mental Health and Neuroscience, Maastricht University, Maastricht, Netherlands, 4Department of Radiation Oncology (MAASTRO), GROW – School for Oncology and Developmental Biology, Maastricht University Medical Center, Maastricht, Netherlands, 5Department of Electrical Engineering, Eindhoven University of Technology, Eindhoven, Netherlands
Synopsis
Blood-brain barrier (BBB) disruption underlies the origin of many brain disorders. Measuring subtle BBB leakage of gadolinium-based contrast agents is commonly performed by a continuous and time-consuming dynamic MRI protocol. However, BBB leakage is subtle, for which discrete sampling at strategic time-points might be sufficient. This study explores the feasibility of the time-efficient interleaved protocol by applying it to brain tumor patients. We were able to measure high permeability in a craniopharyngioma and low permeability in a low-grade tumor and in healthy tissue. The time-efficient protocol is promising and will be further evaluated in more patients.
Introduction
The blood-brain barrier (BBB) is important for maintaining homeostasis in the brain1. When the BBB is disrupted, unwanted biomolecules can freely cross the barrier and leak into the brain parenchyma. The commonly used imaging method to quantify BBB permeability is dynamic contrast-enhanced MRI (DCE-MRI)2. During DCE-MRI, a contrast agent is injected into the vascular system, while continuously acquiring T1-weighted images preferably with a high temporal resolution and a long measurement period (>25min). However, subtle BBB leakage is a slow process and does not require this high sampling rate3. The proposed method consists of a limited number of post-contrast T1-weighted images that are sparsely sampled over time, leaving more time in between for other MRI scans. This is clinically attractive, since it can easily be added to MRI protocols without significantly increasing the scan time. Furthermore, the post-contrast T1-weighted images have better signal-to-noise ratios and the BBB leakage map has a higher spatial resolution, facilitating the detection of BBB leakage in small brain structures. The aim of this study was to explore the feasibility of the novel protocol, by applying it to patients with a brain tumor.Method
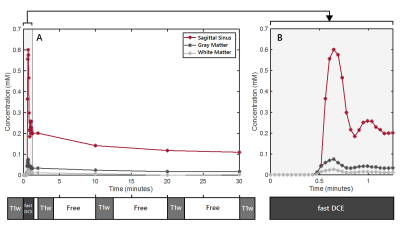
Subjects: Two patients (males, age 66 and 60 years) with a brain tumor lesion (WHO II grade glial tumor in the right hemisphere and recurrent craniopharyngioma) were included.MRI acquisition: Imaging was performed on an 3T MRI system (Achieva TX, Philips Healthcare, Best, the Netherlands) using a 32-element head coil. The protocol for the novel BBB method consisted of one pre-contrast and four post-contrast T1-weighted sequences (Turbo field echo with inversion pulse; TR/TI/TE=6.7/1060/3.0ms; field of view (FOV): 240x240x171mm3; reconstructed voxel size: 0.36x0.36x1.0mm3; Compressed SENSE reduction factor: 4.5; acquisition time=2:19min). The post-contrast scans were acquired at 2, 10, 20 and 30 minutes after contrast agent injection (gadobutrol, dose 0.1mmol Gd/kg, infusion rate: 3mL/s). Especially, the latter scans are important for detecting subtle BBB leakage rates, since they allow the contrast agent more time to accumulate in the extravascular extracellular space3. A fast DCE sequence (saturation recovery gradient recalled sequence; 90° non-selective saturation prepulse with a time delay of 120ms; TR/TE=5.3/2.5ms; FOV: 156x156x55mm3; reconstructed voxel size of 1.0x1.0x5.0mm3; time interval: 2.7s; 30 volumes) was applied during bolus injection to measure the arrival and initial blood circulation of the contrast agent and to obtain the first part of the vascular input function (VIF).
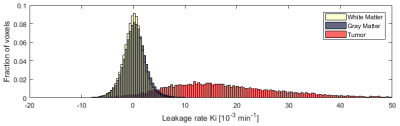
Image analysis: Whole cerebrum white and gray matter regions were obtained by automated segmentation (Freesurfer, v6.0.04), with manual adjustment. The tumor tissue was manually delineated under supervision of an experienced neuroradiologist. Motion correction was performed by registering the post-contrast T1-weighted and the fast DCE images to the pre-contrast T1-weighted image. For conversion of the signal intensity changes to concentration, a pre-contrast T1 relaxation time map (T10) was estimated for the native tissue. The patient-specific VIF was manually retrieved from the superior sagittal sinus. The graphical Patlak approach was used for voxel-wise pharmacokinetic modeling of the concentration in tissue and plasma to obtain the BBB leakage rate (Ki)5. Histograms of the Ki values were calculated from the noisy Ki-maps in gray matter, white matter, and tumor tissue. Hereafter, the histogram approach was applied to extract the detectable leakage volume fraction (VL) and the corresponding noise-level-corrected Ki6.
Results
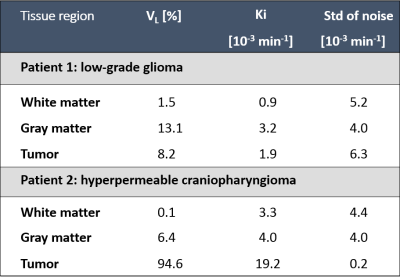
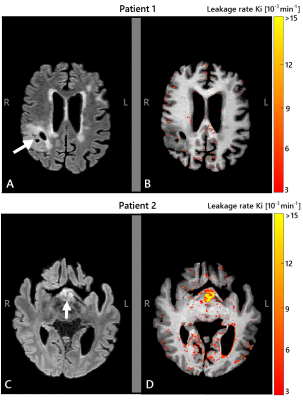
The image acquisition method used for quantification of BBB permeability is shown in figure 1, in which free time frames (white) are shown, which were used to perform other relevant MRI sequences in between. This figure also shows an example of concentration curves over time. Histograms without noise removal are depicted in figure 2. Table 1 shows the VL, the noise-level-corrected Ki values and the standard deviation of the subtracted noise, calculated for white matter, gray matter and tumor tissue. The Ki and the VL in the strongly enhanced craniopharyngioma were higher than in the low-grade tumor. In both subjects, higher VL and Ki were measured for gray matter compared to white matter. The Ki-maps for both patients are shown in figure 3.Discussion
The study shows that, despite the limited temporal resolution after the initial administration of the contrast agent, the time-efficient protocol was able to measure strong leakage in hyperpermeable tumor tissue as well as subtle BBB leakage in healthy appearing tissue. The Ki values are in the same order as found in literature2. Moreover, the usability was also supported by obtaining high tissue permeability in a craniopharyngioma, where strong leakage was expected, and low tissue permeability in a low-grade glioma. The Patlak model tends to underestimate the true Ki values of high leaking tissue, indicating that the BBB leakage in the hyperpermeable tumor is even higher than estimated7. The new DCE-MRI protocol creates 3 time-slots of 6-8 minutes in between for other MRI sequences, of which the effect of contrast agent presence should be evaluated in more detail. Currently, this technique is being applied in more brain tumor patients.Conclusion
The proposed MRI protocol with long-time interleaved sampling for detection of subtle BBB leakage is promising and clinically attractive due to its short total scanning time. This method needs to be further evaluated in a larger group of patients with various levels of BBB disruption.Acknowledgements
No acknowledgement found.References
1Sweeney, M, Zhao, Z, Montagne, A, Nelson, A, & Zlokovic, B. Blood-brain barrier: from physiology to disease and back. Physiological reviews. 2018; 99(1); 21-78
2Raja R, Rosenberg G, Caprihan A. MRI measurements of Blood-Brain Barrier function in dementia: A review of recent studies Neuropharmacology. Neurpharmacology. 2018; 134(B); 259-271
3Barnes S, Ng T, Montagne A, et al. Optimal acquisition and modeling parameters for accurate assessment of low Ktrans blood-brain barrier permeability using dynamic contrast-enhanced MRI. Magn. Reson. Med. 2016; 75(5);1967-1977
4Fischl B, Salat D, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002; 33(3):341-55
5Patlak C, Blasberg R, Fenstermacher J. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Cereb. Blood Flow & Metab.1983; 3(1);1-7
6van de Haar H, Burgmans S, Jansen J, et al. Blood-Brain Barrier Leakage in Patients with Early Alzheimer Disease. Radiology. 2016; 281(2); 527-535
7Cramer S, Larsson H. Accurate determination of blood–brain barrier permeability using dynamic contrast-enhanced T1-weighted MRI: a simulation and in vivo study on healthy subjects and multiple sclerosis patientsCereb. Blood Flow & Metab. 2014; 34(10); 1655-1665
Figures